Engineering genetic circuit interactions within and between synthetic minimal cells
- PMID: 28430194
- PMCID: PMC5407321
- DOI: 10.1038/nchem.2644
Engineering genetic circuit interactions within and between synthetic minimal cells
Abstract
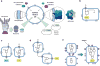
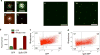
Genetic circuits and reaction cascades are of great importance for synthetic biology, biochemistry and bioengineering. An open question is how to maximize the modularity of their design to enable the integration of different reaction networks and to optimize their scalability and flexibility. One option is encapsulation within liposomes, which enables chemical reactions to proceed in well-isolated environments. Here we adapt liposome encapsulation to enable the modular, controlled compartmentalization of genetic circuits and cascades. We demonstrate that it is possible to engineer genetic circuit-containing synthetic minimal cells (synells) to contain multiple-part genetic cascades, and that these cascades can be controlled by external signals as well as inter-liposomal communication without crosstalk. We also show that liposomes that contain different cascades can be fused in a controlled way so that the products of incompatible reactions can be brought together. Synells thus enable a more modular creation of synthetic biology cascades, an essential step towards their ultimate programmability.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

