Effects of Zika Virus Strain and Aedes Mosquito Species on Vector Competence
- PMID: 28430564
- PMCID: PMC5512477
- DOI: 10.3201/eid2307.161633
Effects of Zika Virus Strain and Aedes Mosquito Species on Vector Competence
Abstract
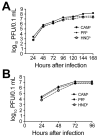
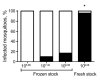
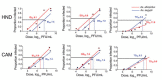
In the Western Hemisphere, Zika virus is thought to be transmitted primarily by Aedes aegypti mosquitoes. To determine the extent to which Ae. albopictus mosquitoes from the United States are capable of transmitting Zika virus and the influence of virus dose, virus strain, and mosquito species on vector competence, we evaluated multiple doses of representative Zika virus strains in Ae. aegypti and Ae. albopictus mosquitoes. Virus preparation (fresh vs. frozen) significantly affected virus infectivity in mosquitoes. We calculated 50% infectious doses to be 6.1-7.5 log 10 PFU/mL; minimum infective dose was 4.2 log 10 PFU/mL. Ae. albopictus mosquitoes were more susceptible to infection than Ae. aegypti mosquitoes, but transmission efficiency was higher for Ae. aegypti mosquitoes, indicating a transmission barrier in Ae. albopictus mosquitoes. Results suggest that, although Zika virus transmission is relatively inefficient overall and dependent on virus strain and mosquito species, Ae. albopictus mosquitoes could become major vectors in the Americas.
Keywords: Aedes aegypti; Aedes albopictus; Zika virus; mosquitoes; vector competence; vector-borne infections; viruses.
Figures

Similar articles
-
Competence of Aedes aegypti, Ae. albopictus, and Culex quinquefasciatus Mosquitoes as Zika Virus Vectors, China.Emerg Infect Dis. 2017 Jul;23(7):1085-1091. doi: 10.3201/eid2307.161528. Epub 2017 Jul 15. Emerg Infect Dis. 2017. PMID: 28430562 Free PMC article.
-
Vector Competence of Aedes caspius and Ae. albopictus Mosquitoes for Zika Virus, Spain.Emerg Infect Dis. 2019 Feb;25(2):346-348. doi: 10.3201/eid2502.171123. Emerg Infect Dis. 2019. PMID: 30666939 Free PMC article.
-
Vertical Transmission of Zika Virus by Aedes aegypti and Ae. albopictus Mosquitoes.Emerg Infect Dis. 2017 May;23(5):880-882. doi: 10.3201/eid2305.162041. Epub 2017 May 15. Emerg Infect Dis. 2017. PMID: 28277199 Free PMC article.
-
Aedes albopictus (Diptera: Culicidae) and Mosquito-Borne Viruses in the United States.J Med Entomol. 2016 Sep;53(5):1024-8. doi: 10.1093/jme/tjw025. Epub 2016 Apr 25. J Med Entomol. 2016. PMID: 27113107 Review.
-
Aedes albopictus is a competent vector of Zika virus: A meta-analysis.PLoS One. 2019 May 21;14(5):e0216794. doi: 10.1371/journal.pone.0216794. eCollection 2019. PLoS One. 2019. PMID: 31112569 Free PMC article.
Cited by
-
Age-structured vectorial capacity reveals timing, not magnitude of within-mosquito dynamics is critical for arbovirus fitness assessment.Parasit Vectors. 2020 Jun 15;13(1):310. doi: 10.1186/s13071-020-04181-4. Parasit Vectors. 2020. PMID: 32539759 Free PMC article.
-
Naturally infected Aedes aegypti collected during a Zika virus outbreak have viral titres consistent with transmission.Emerg Microbes Infect. 2019;8(1):242-244. doi: 10.1080/22221751.2018.1561157. Emerg Microbes Infect. 2019. PMID: 30866777 Free PMC article. No abstract available.
-
Vector competence of Australian Aedes aegypti and Aedes albopictus for an epidemic strain of Zika virus.PLoS Negl Trop Dis. 2019 Apr 4;13(4):e0007281. doi: 10.1371/journal.pntd.0007281. eCollection 2019 Apr. PLoS Negl Trop Dis. 2019. PMID: 30946747 Free PMC article.
-
Zika virus and temperature modulate Elizabethkingia anophelis in Aedes albopictus.Parasit Vectors. 2021 Nov 12;14(1):573. doi: 10.1186/s13071-021-05069-7. Parasit Vectors. 2021. PMID: 34772442 Free PMC article.
-
Modeling intra-mosquito dynamics of Zika virus and its dose-dependence confirms the low epidemic potential of Aedes albopictus.PLoS Pathog. 2020 Dec 31;16(12):e1009068. doi: 10.1371/journal.ppat.1009068. eCollection 2020 Dec. PLoS Pathog. 2020. PMID: 33382858 Free PMC article.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

