Antiangiogenesis and gene aberration-related therapy may improve overall survival in patients with concurrent KRAS and TP53 hotspot mutant cancer
- PMID: 28430579
- PMCID: PMC5464912
- DOI: 10.18632/oncotarget.16840
Antiangiogenesis and gene aberration-related therapy may improve overall survival in patients with concurrent KRAS and TP53 hotspot mutant cancer
Abstract
Purpose: Genetic alterations such as activating KRAS and/or inactivating TP53 are thought to be the most common drivers to tumorigenesis. Therefore, we assessed phase I cancer patients with KRAS+/TP53+ mutations.
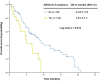
Results: Approximately 8% of patients referred to phase I clinical trials harbored concurrent KRAS and TP53 mutations. Patients who received a phase I trial therapy (n = 57) had a median OS of 12 months, compared with 4.6 months in those who were not treated (n = 106; p = 0.003). KRAS G13 and TP53 R273 mutations were associated with poor overall survival (OS), while antiangiogenesis and gene aberration-related therapies were associated with prolonged OS. A prognostic model using neutrophilia, thrombocytosis, hypoalbuminemia, body mass index <30 kg/m2, and the absence of lung metastasis was established and validated. Phase I cancer patients in the low-risk group had a median OS of 16.6 months compared with 5.4 months in the high-risk group (p < 0.001). Untreated patients in the low-risk group had a median OS of 6.7 months compared with 3.6 months in the high-risk group (p = 0.033).
Experimental design: We analyzed 163 consecutive patients with advanced KRAS+/TP53+ mutant cancer who were referred to phase I clinical trials, to identify molecular aberrations, clinical characteristics, survivals, and potentially effective treatment regimens.
Conclusions: This study provided preliminary evidence that besides modulation of the proinflammatory state, antiangiogensis and concomitant gene aberration-related therapies may improve the treatment of KRAS+/TP53+ mutant cancer.
Keywords: KRAS; TP53; chronic inflammation; gene aberration-related therapy; phase I trial.
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Figures



Similar articles
-
Clinical characteristics and outcomes of phase I cancer patients with CCNE1 amplification: MD Anderson experiences.Sci Rep. 2022 May 24;12(1):8701. doi: 10.1038/s41598-022-12669-5. Sci Rep. 2022. PMID: 35610322 Free PMC article. Review.
-
Phase I study of pazopanib and vorinostat: a therapeutic approach for inhibiting mutant p53-mediated angiogenesis and facilitating mutant p53 degradation.Ann Oncol. 2015 May;26(5):1012-1018. doi: 10.1093/annonc/mdv066. Epub 2015 Feb 10. Ann Oncol. 2015. PMID: 25669829 Free PMC article. Clinical Trial.
-
Effect of Coexisting KRAS and TP53 Mutations in Patients Treated With Chemotherapy for Non-small-cell Lung Cancer.Clin Lung Cancer. 2019 May;20(3):e338-e345. doi: 10.1016/j.cllc.2018.12.009. Epub 2018 Dec 19. Clin Lung Cancer. 2019. PMID: 30770327
-
Sidedness and TP53 mutations impact OS in anti-EGFR but not anti-VEGF treated mCRC - an analysis of the KRAS registry of the AGMT (Arbeitsgemeinschaft Medikamentöse Tumortherapie).BMC Cancer. 2018 Jan 3;18(1):11. doi: 10.1186/s12885-017-3955-4. BMC Cancer. 2018. PMID: 29298682 Free PMC article.
-
Combining TP53 mutation and isoform has the potential to improve clinical practice.Pathology. 2024 Jun;56(4):473-483. doi: 10.1016/j.pathol.2024.02.003. Epub 2024 Mar 19. Pathology. 2024. PMID: 38594116 Review.
Cited by
-
Opposing Roles of IGFBP-3 and Heparanase in Regulating A549 Lung Cancer Cell Survival.Cells. 2022 Nov 8;11(22):3533. doi: 10.3390/cells11223533. Cells. 2022. PMID: 36428962 Free PMC article.
-
Clinical characteristics and outcomes of phase I cancer patients with CCNE1 amplification: MD Anderson experiences.Sci Rep. 2022 May 24;12(1):8701. doi: 10.1038/s41598-022-12669-5. Sci Rep. 2022. PMID: 35610322 Free PMC article. Review.
-
Bicentric lesion of colon cancer with postoperative fever: A case report.Oncol Lett. 2024 Aug 13;28(4):497. doi: 10.3892/ol.2024.14630. eCollection 2024 Oct. Oncol Lett. 2024. PMID: 39211303 Free PMC article.
-
Phase I studies of vorinostat with ixazomib or pazopanib imply a role of antiangiogenesis-based therapy for TP53 mutant malignancies.Sci Rep. 2020 Feb 20;10(1):3080. doi: 10.1038/s41598-020-58366-z. Sci Rep. 2020. PMID: 32080210 Free PMC article. Clinical Trial.
-
Mutated TP53 is a marker of increased VEGF expression: analysis of 7,525 pan-cancer tissues.Cancer Biol Ther. 2020;21(1):95-100. doi: 10.1080/15384047.2019.1665956. Epub 2019 Sep 29. Cancer Biol Ther. 2020. PMID: 31564192 Free PMC article.
References
-
- Singh H, Longo DL, Chabner BA. Improving Prospects for Targeting RAS. Journal of clinical oncology. 2015;33:3650–3659. - PubMed
-
- Okumura S, Janne PA. Molecular pathways: the basis for rational combination using MEK inhibitors in KRAS-mutant cancers. Clinical cancer research. 2014;20:4193–4199. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

