Fluoxetine attenuates the impairment of spatial learning ability and prevents neuron loss in middle-aged APPswe/PSEN1dE9 double transgenic Alzheimer's disease mice
- PMID: 28430602
- PMCID: PMC5438600
- DOI: 10.18632/oncotarget.15398
Fluoxetine attenuates the impairment of spatial learning ability and prevents neuron loss in middle-aged APPswe/PSEN1dE9 double transgenic Alzheimer's disease mice
Abstract
Selective serotonin reuptake inhibitors (SSRIs) have been reported to increase cognitive performance in some clinical studies of Alzheimer's disease (AD). However, there is a lack of evidence supporting the efficacy of SSRIs as cognition enhancers in AD, and the role of SSRIs as a treatment for AD remains largely unclear. Here, we characterized the impact of fluoxetine (FLX), a well-known SSRI, on neurons in the dentate gyrus (DG) and in CA1 and CA3 of the hippocampus of middle-aged (16 to 17 months old) APPswe/PSEN1dE9 (APP/PS1) transgenic AD model mice. We found that intraperitoneal (i.p.) injection of FLX (10 mg/kg/day) for 5 weeks effectively alleviated the impairment of spatial learning ability in middle-aged APP/PS1 mice as evaluated using the Morris water maze. More importantly, the number of neurons in the hippocampal DG was significantly increased by FLX. Additionally, FLX reduced the deposition of beta amyloid, inhibited GSK-3β activity and increased the level of β-catenin in middle-aged APP/PS1 mice. Collectively, the results of this study indicate that FLX delayed the progression of neuronal loss in the hippocampal DG in middle-aged AD mice, and this effect may underlie the FLX-induced improvement in learning ability. FLX may therefore serve as a promising therapeutic drug for AD.
Keywords: APP/PS1 mice; Alzheimer’s disease; Gerotarget; cognition; fluoxetine; neuron.
Conflict of interest statement
The authors confirm that this report has no biomedical financial interests or potential conflicts of interest.
Figures


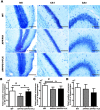
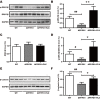


References
-
- Simic G, Kostovic I, Winblad B, Bogdanovic N. Volume and number of neurons of the human hippocampal formation in normal aging and Alzheimer’s disease. J Comp Neurol. 1997;379:482–494. - PubMed
-
- Bloom GS. Amyloid-beta and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71:505–508. - PubMed
-
- Susanto TA, Pua EP, Zhou J, Alzheimer’s Disease Neuroimaging Initiative Cognition, brain atrophy, and cerebrospinal fluid biomarkers changes from preclinical to dementia stage of Alzheimer’s disease and the influence of apolipoprotein e. J Alzheimers Dis. 2015;45:253–268. - PubMed
-
- Wai MS, Liang Y, Shi C, Cho EY, Kung HF, Yew DT. Co-localization of hyperphosphorylated tau and caspases in the brainstem of Alzheimer’s disease patients. Biogerontology. 2009;10:457–469. - PubMed
-
- Gomez-Isla T, Hollister R, West H, Mui S, Growdon JH, Petersen RC, Parisi JE, Hyman BT. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer’s disease. Ann Neurol. 1997;41:17–24. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

