Characterization of pressure-mediated vascular tone in resistance arteries from bile duct-ligated rats
- PMID: 28430609
- PMCID: PMC5458161
- DOI: 10.18632/oncotarget.15409
Characterization of pressure-mediated vascular tone in resistance arteries from bile duct-ligated rats
Abstract
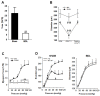
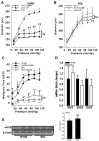
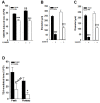
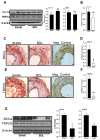
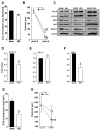
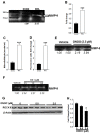
In cirrhosis, changes in pressure-mediated vascular tone, a key determinant of systemic vascular resistance (SVR), are unknown. To address this gap in knowledge, we assessed ex vivo dynamics of pressurized mesenteric resistance arteries (diameter ~ 260 μm) from bile duct-ligated (BDL) and sham-operated (SHAM) rats and determined the underlying mechanisms. At isobaric intraluminal pressure (70 mmHg) as well as with step-wise increase in pressure (10-110 mmHg), arteries from SHAM-rats constricted more than BDL-rats, and had reduced luminal area. In both groups, incubation with LNAME (a NOS inhibitor) had no effect on pressure-mediated tone, and expression of NOS isoforms were similar. TEA, which enhances Ca2+ influx, augmented arterial tone only in SHAM-rats, with minimal effect in those from BDL-rats that was associated with reduced expression of Ca2+ channel TRPC6. In permeabilized arteries, high-dose Ca2+ and γGTP enhanced the vascular tone, which remained lower in BDL-rats that was associated with reduced ROCK2 and pMLC expression. Further, compared to SHAM-rats, in BDL-rats, arteries had reduced collagen expression which was associated with increased expression and activity of MMP-9. BDL-rats also had increased plasma reactive oxygen species (ROS). In vascular smooth muscle cells in vitro, peroxynitrite enhanced MMP-9 activity and reduced ROCK2 expression. These data provide evidence that in cirrhosis, pressure-mediated tone is reduced in resistance arteries, and suggest that circulating ROS play a role in reducing Ca2+ sensitivity and enhancing elasticity to induce arterial adaptations. These findings provide insights into mechanisms underlying attenuated SVR in cirrhosis.
Keywords: Pathology Section; cirrhosis; mesenteric arteries; myogenic tone; portal hypertension; vascular dysfunction.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

Similar articles
-
Intrahepatic upregulation of RhoA and Rho-kinase signalling contributes to increased hepatic vascular resistance in rats with secondary biliary cirrhosis.Gut. 2006 Sep;55(9):1296-305. doi: 10.1136/gut.2005.081059. Epub 2006 Feb 21. Gut. 2006. PMID: 16492715 Free PMC article.
-
Enhanced role for RhoA-associated kinase in adrenergic-mediated vasoconstriction in gracilis arteries from obese Zucker rats.Am J Physiol Regul Integr Comp Physiol. 2006 Jan;290(1):R154-61. doi: 10.1152/ajpregu.00245.2005. Epub 2005 Sep 1. Am J Physiol Regul Integr Comp Physiol. 2006. PMID: 16141308
-
iNOS expression in vascular resident macrophages contributes to circulatory dysfunction of splanchnic vascular smooth muscle contractions in portal hypertensive rats.Am J Physiol Heart Circ Physiol. 2011 Mar;300(3):H1021-31. doi: 10.1152/ajpheart.00563.2009. Epub 2010 Dec 30. Am J Physiol Heart Circ Physiol. 2011. PMID: 21193589
-
Reactive oxygen species and RhoA signaling in vascular smooth muscle: role in chronic hypoxia-induced pulmonary hypertension.Adv Exp Med Biol. 2010;661:355-73. doi: 10.1007/978-1-60761-500-2_23. Adv Exp Med Biol. 2010. PMID: 20204742 Review.
-
Arterial myogenic properties of the spontaneously hypertensive rat.Exp Physiol. 2002 Sep;87(5):527-34. doi: 10.1113/eph8702399. Exp Physiol. 2002. PMID: 12481926 Review.
Cited by
-
Gender differences in vascular reactivity of mesenteric arterioles in portal hypertensive and non-portal hypertensive rats.World J Gastroenterol. 2019 Oct 21;25(39):5953-5960. doi: 10.3748/wjg.v25.i39.5953. World J Gastroenterol. 2019. PMID: 31660032 Free PMC article.
-
Rho-kinase inhibitor coupled to peptide-modified albumin carrier reduces portal pressure and increases renal perfusion in cirrhotic rats.Sci Rep. 2019 Feb 19;9(1):2256. doi: 10.1038/s41598-019-38678-5. Sci Rep. 2019. PMID: 30783172 Free PMC article.
-
Downregulation of Ca2+-Activated Cl- Channel TMEM16A Mediated by Angiotensin II in Cirrhotic Portal Hypertensive Mice.Front Pharmacol. 2022 Mar 16;13:831311. doi: 10.3389/fphar.2022.831311. eCollection 2022. Front Pharmacol. 2022. PMID: 35370660 Free PMC article.
-
Gender-specific differential expression of exosomal miRNA in synovial fluid of patients with osteoarthritis.Sci Rep. 2017 May 17;7(1):2029. doi: 10.1038/s41598-017-01905-y. Sci Rep. 2017. PMID: 28515465 Free PMC article.
References
-
- Moller S, Christensen E, Henriksen JH. Continuous blood pressure monitoring in cirrhosis. Relations to splanchnic and systemic haemodynamics. J Hepatol. 1997;27:284–294. - PubMed
-
- Bosch J, Garcia-Pagan JC. Complications of cirrhosis. I. Portal hypertension. J Hepatol. 2000;32((1 Suppl)):141–156. - PubMed
-
- Forrest EH, Jalan R, Hayes PC. Review article: renal circulatory changes in cirrhosis—pathogenesis and therapeutic prospects. Aliment Pharmacol Ther. 1996;10:219–231. - PubMed
-
- Tazi KA, Barriere E, Moreau R, Heller J, Sogni P, Pateron D, Poirel O, Lebrec D. Role of shear stress in aortic eNOS up-regulation in rats with biliary cirrhosis. Gastroenterology. 2002;122:1869–1877. - PubMed
-
- Atucha NM, Ortiz MC, Fortepiani LA, Nadal FJ, Martinez-Prieto C, Garcia-Estan J. Mesenteric hyporesponsiveness in cirrhotic rats with ascites: role of cGMP and K+ channels. Clin Sci (Lond) 2000;99:455–460. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

