Serious adverse events of cell therapy for respiratory diseases: a systematic review and meta-analysis
- PMID: 28430622
- PMCID: PMC5444761
- DOI: 10.18632/oncotarget.15426
Serious adverse events of cell therapy for respiratory diseases: a systematic review and meta-analysis
Abstract
Background: Cell therapy holds the most promising for acute and chronic deleterious respiratory diseases. However, the safety and tolerance for lung disorders are controversy.
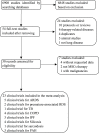
Methods: We undertook a systematic review and meta-analyses of all 23 clinical studies of cell therapy. The outcomes were odds ratio (OR), risk difference (RD), Peto OR, relative risk, and mean difference of serious adverse events.
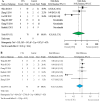
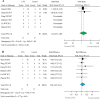
Results: 342 systemic infusions and 57 bronchial instillations (204 recipients) of cells were analyzed for acute respiratory distress syndrome (ARDS), bronchopulmonary dysplasia, pulmonary arterial hypertension, silicosis, sarcoidosis, extensively drug-resistant tuberculosis, chronic obstructive pulmonary diseases (COPD), and idiopathic pulmonary fibrosis. The frequency of death in adults from any causes was 71 and 177 per 1,000 for cell therapy and controls, respectively, with an OR of 0.31 (95% CI: 0.03, 3.76) and RD of -0.22 (95% CI: -0.53, 0.09). No significant difference was found for ARDS and COPD. The frequency of deaths and non-fatal serious adverse events of 17 open studies were similar to those of randomized controlled trials. Moreover, serious adverse events of allogenic cells were greater than autologous preparations, as shown by frequency, OR and RD.
Conclusions: We conclude that either infusion or instillation of mesenchymal stem stromal or progenitor cells are well tolerated without serious adverse events causally related to cell treatment. Cell therapy has not been associated with significant changes in spirometry, immune function, cardiovascular activity, and the quality of life.
Keywords: cell therapy; meta-analysis; respiratory diseases; serious adverse events; systematic review.
Conflict of interest statement
The authors have no conflicts of interest to report.
Figures
Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Prophylactic antibiotics for adults with chronic obstructive pulmonary disease: a network meta-analysis.Cochrane Database Syst Rev. 2021 Jan 15;1(1):CD013198. doi: 10.1002/14651858.CD013198.pub2. Cochrane Database Syst Rev. 2021. PMID: 33448349 Free PMC article.
-
Magnesium sulfate for acute exacerbations of chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2022 May 26;5(5):CD013506. doi: 10.1002/14651858.CD013506.pub2. Cochrane Database Syst Rev. 2022. PMID: 35616126 Free PMC article.
-
Antidepressants for pain management in adults with chronic pain: a network meta-analysis.Health Technol Assess. 2024 Oct;28(62):1-155. doi: 10.3310/MKRT2948. Health Technol Assess. 2024. PMID: 39367772 Free PMC article.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
Cited by
-
Photopolymerizable Hydrogel for Enhanced Intramyocardial Vascular Progenitor Cell Delivery and Post-Myocardial Infarction Healing.Adv Healthc Mater. 2023 Nov;12(29):e2301581. doi: 10.1002/adhm.202301581. Epub 2023 Aug 31. Adv Healthc Mater. 2023. PMID: 37611321 Free PMC article.
-
A phase I study of autologous mesenchymal stromal cells for severe steroid-dependent nephrotic syndrome.JCI Insight. 2023 Sep 22;8(18):e169424. doi: 10.1172/jci.insight.169424. JCI Insight. 2023. PMID: 37561590 Free PMC article. Clinical Trial.
-
Associations of D-Dimer on Admission and Clinical Features of COVID-19 Patients: A Systematic Review, Meta-Analysis, and Meta-Regression.Front Immunol. 2021 May 7;12:691249. doi: 10.3389/fimmu.2021.691249. eCollection 2021. Front Immunol. 2021. PMID: 34025688 Free PMC article.
-
Mesenchymal stem cells as living anti-inflammatory therapy for COVID-19 related acute respiratory distress syndrome.World J Stem Cells. 2020 Oct 26;12(10):1067-1079. doi: 10.4252/wjsc.v12.i10.1067. World J Stem Cells. 2020. PMID: 33178392 Free PMC article. Review.
-
Mesenchymal stem cells for the prevention and treatment of bronchopulmonary dysplasia in preterm infants.Cochrane Database Syst Rev. 2017 Nov 10;11(11):CD011932. doi: 10.1002/14651858.CD011932.pub2. Cochrane Database Syst Rev. 2017. PMID: 29125893 Free PMC article.
References
-
- Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, Ranieri M, Rubenfeld G, Thompson BT, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. - DOI - PubMed
-
- McIntyre LA, Moher D, Fergusson DA, Sullivan KJ, Mei SH, Lalu M, Marshall J, McLeod M, Griffin G, Grimshaw J, Turgeon A, Avey MT, Rudnicki MA, et al. Efficacy of mesenchymal stromal cell therapy for acute lung injury in preclinical animal models: a systematic review. PLoS One. 2016;11:e0147170. doi: 10.1371/journal.pone.0147170. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

