Unsuccessful mitosis in multicellular tumour spheroids
- PMID: 28430635
- PMCID: PMC5438690
- DOI: 10.18632/oncotarget.15673
Unsuccessful mitosis in multicellular tumour spheroids
Abstract
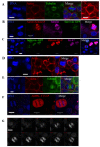
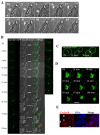
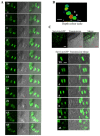
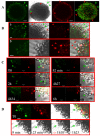
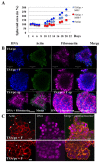
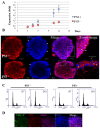
Multicellular spheroids are very attractive models in oncology because they mimic the 3D organization of the tumour cells with their microenvironment. We show here using 3 different cell types (mammary TSA/pc, embryonic kidney Hek293 and cervical cancer HeLa), that when the cells are growing as spheroids the frequency of binucleated cells is augmented as occurs in some human tumours.We therefore describe mitosis in multicellular spheroids by following mitotic markers and by time-lapse experiments. Chromosomes alignment appears to be correct on the metaphasic plate and the passenger complex is well localized on centromere. Moreover aurora kinases are fully active and histone H3 is phosphorylated on Ser 10. Consequently, the mitotic spindle checkpoint is satisfied and, anaphase proceeds as illustrated by the transfer of survivin on the spindle and by the segregation of the two lots of chromosomes. However, the segregation plane is not well defined and oscillations of the dividing cells are observed. Finally, cytokinesis fails and the absence of separation of the two daughter cells gives rise to binucleated cells.Division orientation is specified during interphase and persists throughout mitosis. Our data indicate that the cancer cells, in multicellular spheroids, lose their ability to regulate their orientation, a feature commonly encountered in tumours.Moreover, multicellular spheroid expansion is still sensitive to mitotic drugs as pactlitaxel and aurora kinase inhibitors. The spheroids thus represent a highly relevant model for studying drug efficiency in tumours.
Keywords: cytokinesis; mitosis; mitotic drug; spheroid; tetraploid cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Unger C, Kramer N, Walzl A, Scherzer M, Hengstschläger M, Dolznig H. Modeling human carcinomas: physiologically relevant 3D models to improve anti-cancer drug development. Adv Drug Deliv Rev. 2014. pp. 79–80. 50-67. - PubMed
-
- Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130:601–10. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

