Identification of a novel PD-L1 positive solid tumor transplantable in HLA-A*0201/DRB1*0101 transgenic mice
- PMID: 28430664
- PMCID: PMC5564740
- DOI: 10.18632/oncotarget.16900
Identification of a novel PD-L1 positive solid tumor transplantable in HLA-A*0201/DRB1*0101 transgenic mice
Abstract
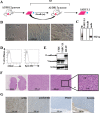
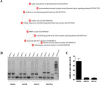
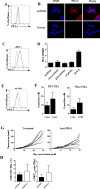
HLA-A*0201/DRB1*0101 transgenic mice (A2/DR1 mice) have been developed to study the immunogenicity of tumor antigen-derived T cell epitopes. To extend the use and application of this mouse model in the field of antitumor immunotherapy, we described a tumor cell line generated from a naturally occurring tumor in A2/DR1 mouse named SARC-L1. Histological and genes signature analysis supported the sarcoma origin of this cell line. While SARC-L1 tumor cells lack HLA-DRB1*0101 expression, a very low expression of HLA-A*0201 molecules was found on these cells. Furthermore they also weakly but constitutively expressed the programmed death-ligand 1 (PD-L1). Interestingly both HLA-A*0201 and PD-L1 expressions can be increased on SARC-L1 after IFN-γ exposure in vitro. We also obtained two genetically modified cell lines highly expressing either HLA-A*0201 or both HLA-A*0201/ HLA-DRB1*0101 molecules referred as SARC-A2 and SARC-A2DR1 respectively. All the SARC-L1-derived cell lines induced aggressive subcutaneous tumors in A2DR1 mice in vivo. The analysis of SARC-L1 tumor microenvironment revealed a strong infiltration by T cells expressing inhibitory receptors such as PD-1 and TIM-3. Finally, we found that SARC-L1 is sensitive to several drugs commonly used to treat sarcoma and also susceptible to anti-PD-L1 monoclonal antibody therapy in vivo. Collectively, we described a novel syngeneic tumor model A2/DR1 mice that could be used as preclinical tool for the evaluation of antitumor immunotherapies.
Keywords: HLA transgenic mouse; PD-L1; T cells; cancer immunotherapy; sarcoma.
Conflict of interest statement
The authors have declared no conflict of interest.
Figures



Similar articles
-
A human programmed death-ligand 1-expressing mouse tumor model for evaluating the therapeutic efficacy of anti-human PD-L1 antibodies.Sci Rep. 2017 Feb 16;7:42687. doi: 10.1038/srep42687. Sci Rep. 2017. PMID: 28202921 Free PMC article.
-
Phosphatidylserine-targeting antibodies augment the anti-tumorigenic activity of anti-PD-1 therapy by enhancing immune activation and downregulating pro-oncogenic factors induced by T-cell checkpoint inhibition in murine triple-negative breast cancers.Breast Cancer Res. 2016 May 11;18(1):50. doi: 10.1186/s13058-016-0708-2. Breast Cancer Res. 2016. PMID: 27169467 Free PMC article.
-
In Vivo Antitumor Activity of the PD-1/PD-L1 Inhibitor SCL-1 in Various Mouse Tumor Models.In Vivo. 2025 Jan-Feb;39(1):80-95. doi: 10.21873/invivo.13805. In Vivo. 2025. PMID: 39740910 Free PMC article.
-
What does PD-L1 positive or negative mean?J Exp Med. 2016 Dec 12;213(13):2835-2840. doi: 10.1084/jem.20161462. Epub 2016 Nov 30. J Exp Med. 2016. PMID: 27903604 Free PMC article. Review.
-
Clinical implications of tumor-intrinsic mechanisms regulating PD-L1.Sci Transl Med. 2019 Feb 6;11(478):eaav4810. doi: 10.1126/scitranslmed.aav4810. Sci Transl Med. 2019. PMID: 30728286 Review.
Cited by
-
Cancer vaccines: designing artificial synthetic long peptides to improve presentation of class I and class II T cell epitopes by dendritic cells.Oncoimmunology. 2019 Jan 17;8(4):e1560919. doi: 10.1080/2162402X.2018.1560919. eCollection 2019. Oncoimmunology. 2019. PMID: 30906653 Free PMC article.
-
Role of Human Leukocyte Antigen System as A Predictive Biomarker for Checkpoint-Based Immunotherapy in Cancer Patients.Int J Mol Sci. 2020 Oct 2;21(19):7295. doi: 10.3390/ijms21197295. Int J Mol Sci. 2020. PMID: 33023239 Free PMC article. Review.
-
Stereotactic Body Radiotherapy and Immunotherapy for Older Patients with Oligometastases: A Proposed Paradigm by the International Geriatric Radiotherapy Group.Cancers (Basel). 2022 Dec 30;15(1):244. doi: 10.3390/cancers15010244. Cancers (Basel). 2022. PMID: 36612239 Free PMC article.
-
High Therapeutic Efficacy of a New Survivin LSP-Cancer Vaccine Containing CD4+ and CD8+ T-Cell Epitopes.Front Oncol. 2018 Nov 13;8:517. doi: 10.3389/fonc.2018.00517. eCollection 2018. Front Oncol. 2018. PMID: 30483475 Free PMC article.
-
Mimicry-based strategy between human and commensal antigens for the development of a new family of immune therapies for cancer.J Immunother Cancer. 2025 Feb 20;13(2):e010192. doi: 10.1136/jitc-2024-010192. J Immunother Cancer. 2025. PMID: 39979071 Free PMC article.
References
-
- Adotévi O, Mollier K, Neuveut C, Cardinaud S, Boulanger E, Mignen B, Fridman W-H, Zanetti M, Charneau P, Tartour E, Lemonnier F, Langlade-Demoyen P. Immunogenic HLA-B*0702-Restricted Epitopes Derived from Human Telomerase Reverse Transcriptase That Elicit Antitumor Cytotoxic T-Cell Responses. Clin Cancer Res. 2006;12:3158–67. - PubMed
-
- Boucherma R, Kridane-Miledi H, Bouziat R, Rasmussen M, Gatard T, Langa-Vives F, Lemercier B, Lim A, Bérard M, Benmohamed L, Buus S, Rooke R, Lemonnier FA. HLA-A*01: 03, HLA-A*24: 02, HLA-B*08: 01, HLA-B*27: 05, HLA-B*35: 01, HLA-B*44: 02, and HLA-C*07: 01 monochain transgenic/H-2 class I null mice: novel versatile preclinical models of human T cell responses. J Immunol. 1950;2013;191:583–93. - PMC - PubMed
-
- Pascolo S. HLA class I transgenic mice: development, utilisation and improvement. Expert Opin Biol Ther. 2005;5:919–38. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

