The burden of trisomy 21 disrupts the proteostasis network in Down syndrome
- PMID: 28430800
- PMCID: PMC5400264
- DOI: 10.1371/journal.pone.0176307
The burden of trisomy 21 disrupts the proteostasis network in Down syndrome
Abstract
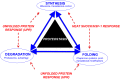
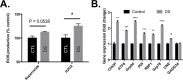
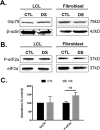
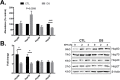
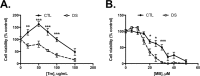
Down syndrome (DS) is a genetic disorder caused by trisomy of chromosome 21. Abnormalities in chromosome number have the potential to lead to disruption of the proteostasis network (PN) and accumulation of misfolded proteins. DS individuals suffer from several comorbidities, and we hypothesized that disruption of proteostasis could contribute to the observed pathology and decreased cell viability in DS. Our results confirm the presence of a disrupted PN in DS, as several of its elements, including the unfolded protein response, chaperone system, and proteasomal degradation exhibited significant alterations compared to euploid controls in both cell and mouse models. Additionally, when cell models were treated with compounds that promote disrupted proteostasis, we observed diminished levels of cell viability in DS compared to controls. Collectively our findings provide a cellular-level characterization of PN dysfunction in DS and an improved understanding of the potential pathogenic mechanisms contributing to disrupted cellular physiology in DS. Lastly, this study highlights the future potential of designing therapeutic strategies that mitigate protein quality control dysfunction.
Conflict of interest statement
Figures



Similar articles
-
SNARE proteins rescue impaired autophagic flux in Down syndrome.PLoS One. 2019 Nov 12;14(11):e0223254. doi: 10.1371/journal.pone.0223254. eCollection 2019. PLoS One. 2019. PMID: 31714914 Free PMC article.
-
Lipid peroxidation in Down syndrome caused by regular trisomy 21, trisomy 21 by Robertsonian translocation and mosaic trisomy 21.Clin Chem Lab Med. 2007;45(1):59-62. doi: 10.1515/CCLM.2007.011. Clin Chem Lab Med. 2007. PMID: 17243916
-
YAC and cosmid FISH mapping of an unbalanced chromosomal translocation causing partial trisomy 21 and Down syndrome.Hum Genet. 1996 Oct;98(4):460-6. doi: 10.1007/s004390050240. Hum Genet. 1996. PMID: 8792823
-
Molecular genetic analysis of Down syndrome.Hum Genet. 2009 Jul;126(1):195-214. doi: 10.1007/s00439-009-0696-8. Epub 2009 Jun 13. Hum Genet. 2009. PMID: 19526251 Review.
-
Down syndrome--genetic and nutritional aspects of accompanying disorders.Rocz Panstw Zakl Hig. 2015;66(3):189-94. Rocz Panstw Zakl Hig. 2015. PMID: 26400113 Review.
Cited by
-
Activation of the ISR mediates the behavioral and neurophysiological abnormalities in Down syndrome.Science. 2019 Nov 15;366(6467):843-849. doi: 10.1126/science.aaw5185. Science. 2019. PMID: 31727829 Free PMC article.
-
Comparative analysis of the DYRK1A-SRSF6-TNNT2 pathway in myocardial tissue from individuals with and without Down syndrome.Exp Mol Pathol. 2019 Oct;110:104268. doi: 10.1016/j.yexmp.2019.104268. Epub 2019 Jun 12. Exp Mol Pathol. 2019. PMID: 31201803 Free PMC article.
-
Cellular Stress Associated with Aneuploidy.Dev Cell. 2018 Feb 26;44(4):420-431. doi: 10.1016/j.devcel.2018.02.002. Dev Cell. 2018. PMID: 29486194 Free PMC article. Review.
-
Toxicant-mediated redox control of proteostasis in neurodegeneration.Curr Opin Toxicol. 2019 Feb;13:22-34. doi: 10.1016/j.cotox.2018.12.007. Epub 2018 Dec 28. Curr Opin Toxicol. 2019. PMID: 31602419 Free PMC article.
-
Antioxidants in Down Syndrome: From Preclinical Studies to Clinical Trials.Antioxidants (Basel). 2020 Aug 3;9(8):692. doi: 10.3390/antiox9080692. Antioxidants (Basel). 2020. PMID: 32756318 Free PMC article. Review.
References
-
- Patterson D. Molecular genetic analysis of Down syndrome. Hum Genet. 2009;126(1):195–214. doi: 10.1007/s00439-009-0696-8 - DOI - PubMed
-
- Savva GM, Walker K, Morris JK. The maternal age-specific live birth prevalence of trisomies 13 and 18 compared to trisomy 21 (Down syndrome). Prenat Diagn. 2010;30(1):57–64. doi: 10.1002/pd.2403 - DOI - PubMed
-
- Oromendia AB, Dodgson SE, Amon A. Aneuploidy causes proteotoxic stress in yeast. Genes Dev. 2012;26(24):2696–708. PubMed Central PMCID: PMCPMC3533075. doi: 10.1101/gad.207407.112 - DOI - PMC - PubMed
-
- Stingele S, Stoehr G, Peplowska K, Cox J, Mann M, Storchova Z. Global analysis of genome, transcriptome and proteome reveals the response to aneuploidy in human cells. Mol Syst Biol. 2012;8:608 PubMed Central PMCID: PMCPMC3472693. doi: 10.1038/msb.2012.40 - DOI - PMC - PubMed
-
- Lott IT, Doran E, Nguyen VQ, Tournay A, Movsesyan N, Gillen DL. Down syndrome and dementia: seizures and cognitive decline. Journal of Alzheimer's disease: JAD. 2012;29(1):177–85. PubMed Central PMCID: PMC3406603. doi: 10.3233/JAD-2012-111613 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

