Dual role of the Toxoplasma gondii clathrin adaptor AP1 in the sorting of rhoptry and microneme proteins and in parasite division
- PMID: 28430827
- PMCID: PMC5415223
- DOI: 10.1371/journal.ppat.1006331
Dual role of the Toxoplasma gondii clathrin adaptor AP1 in the sorting of rhoptry and microneme proteins and in parasite division
Abstract
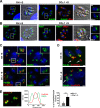
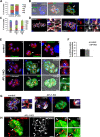
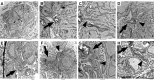
Toxoplasma gondii possesses a highly polarized secretory system, which efficiently assembles de novo micronemes and rhoptries during parasite replication. These apical secretory organelles release their contents into host cells promoting parasite invasion and survival. Using a CreLox-based inducible knock-out strategy and the ddFKBP over-expression system, we unraveled novel functions of the clathrin adaptor complex TgAP1. First, our data indicate that AP1 in T. gondii likely functions as a conserved heterotetrameric complex composed of the four subunits γ, β, μ1, σ1 and interacts with known regulators of clathrin-mediated vesicular budding such as the unique ENTH-domain containing protein, which we named Epsin-like protein (TgEpsL). Disruption of the μ1 subunit resulted in the mis-sorting of microneme proteins at the level of the Trans-Golgi-Network (TGN). Furthermore, we demonstrated that TgAP1 regulates rhoptry biogenesis by activating rhoptry protein exit from the TGN, but also participates in the post-Golgi maturation process of preROP compartments into apically anchored club-shaped mature organelles. For this latter activity, our data indicate a specific functional relationship between TgAP1 and the Rab5A-positive endosome-like compartment. In addition, we unraveled an original role for TgAP1 in the regulation of parasite division. APμ1-depleted parasites undergo normal daughter cell budding and basal complex assembly but fail to segregate at the end of cytokinesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
The role of clathrin in post-Golgi trafficking in Toxoplasma gondii.PLoS One. 2013 Oct 11;8(10):e77620. doi: 10.1371/journal.pone.0077620. eCollection 2013. PLoS One. 2013. PMID: 24147036 Free PMC article.
-
Targeting to rhoptry organelles of Toxoplasma gondii involves evolutionarily conserved mechanisms.Nat Cell Biol. 2000 Jul;2(7):449-56. doi: 10.1038/35017090. Nat Cell Biol. 2000. PMID: 10878811
-
Toxoplasma gondii Syntaxin 6 is required for vesicular transport between endosomal-like compartments and the Golgi complex.Traffic. 2013 Nov;14(11):1166-81. doi: 10.1111/tra.12102. Epub 2013 Sep 12. Traffic. 2013. PMID: 23962112 Free PMC article.
-
Preparing for an invasion: charting the pathway of adhesion proteins to Toxoplasma micronemes.Parasitol Res. 2006 Apr;98(5):389-95. doi: 10.1007/s00436-005-0062-2. Epub 2005 Dec 30. Parasitol Res. 2006. PMID: 16385407 Review.
-
Biogenesis and discharge of the rhoptries: Key organelles for entry and hijack of host cells by the Apicomplexa.Mol Microbiol. 2021 Mar;115(3):453-465. doi: 10.1111/mmi.14674. Mol Microbiol. 2021. PMID: 33368727 Review.
Cited by
-
Toxoplasma gondii's Basal Complex: The Other Apicomplexan Business End Is Multifunctional.Front Cell Infect Microbiol. 2022 Apr 29;12:882166. doi: 10.3389/fcimb.2022.882166. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35573773 Free PMC article.
-
The Toxoplasma micropore mediates endocytosis for selective nutrient salvage from host cell compartments.Nat Commun. 2023 Feb 22;14(1):977. doi: 10.1038/s41467-023-36571-4. Nat Commun. 2023. PMID: 36813769 Free PMC article.
-
Moderate Fluid Shear Stress Could Regulate the Cytoskeleton of Nucleus Pulposus and Surrounding Inflammatory Mediators by Activating the FAK-MEK5-ERK5-cFos-AP1 Signaling Pathway.Dis Markers. 2018 Jun 12;2018:9405738. doi: 10.1155/2018/9405738. eCollection 2018. Dis Markers. 2018. PMID: 30008976 Free PMC article.
-
Stable endocytic structures navigate the complex pellicle of apicomplexan parasites.Nat Commun. 2023 Apr 15;14(1):2167. doi: 10.1038/s41467-023-37431-x. Nat Commun. 2023. PMID: 37061511 Free PMC article.
-
The Plasmodium falciparum Artemisinin Susceptibility-Associated AP-2 Adaptin μ Subunit is Clathrin Independent and Essential for Schizont Maturation.mBio. 2020 Feb 25;11(1):e02918-19. doi: 10.1128/mBio.02918-19. mBio. 2020. PMID: 32098816 Free PMC article.
References
-
- Saadatnia G, Golkar M. A review on human toxoplasmosis. Scand J Infect Dis. 2012. November;44(11):805–14. doi: 10.3109/00365548.2012.693197 - DOI - PubMed
-
- Besteiro S, Dubremetz J-F, Lebrun M. The moving junction of apicomplexan parasites: a key structure for invasion. Cell Microbiol. 2011. June;13(6):797–805. doi: 10.1111/j.1462-5822.2011.01597.x - DOI - PubMed
-
- Lamarque MH, Roques M, Kong-Hap M, Tonkin ML, Rugarabamu G, Marq J-B, et al. Plasticity and redundancy among AMA-RON pairs ensure host cell entry of Toxoplasma parasites. Nat Commun. 2014. June 17;5:4098 doi: 10.1038/ncomms5098 - DOI - PubMed
-
- Carruthers V, Boothroyd JC. Pulling together: an integrated model of Toxoplasma cell invasion. Curr Opin Microbiol. 2007. February;10(1):83–9. doi: 10.1016/j.mib.2006.06.017 - DOI - PubMed
-
- Hunter CA, Sibley LD. Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat Rev Microbiol. 2012. November;10(11):766–78. doi: 10.1038/nrmicro2858 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

