Repurposed drugs targeting eIF2α-P-mediated translational repression prevent neurodegeneration in mice
- PMID: 28430857
- PMCID: PMC5445255
- DOI: 10.1093/brain/awx074
Repurposed drugs targeting eIF2α-P-mediated translational repression prevent neurodegeneration in mice
Abstract
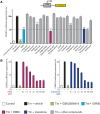
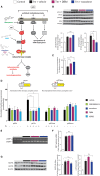
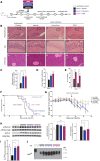
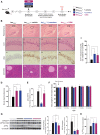
See Mercado and Hetz (doi:10.1093/brain/awx107) for a scientific commentary on this article.Signalling through the PERK/eIF2α-P branch of the unfolded protein response plays a critical role in controlling protein synthesis rates in cells. This pathway is overactivated in brains of patients with Alzheimer's disease and related disorders and has recently emerged as a promising therapeutic target for these currently untreatable conditions. Thus, in mouse models of neurodegenerative disease, prolonged overactivation of PERK/eIF2α-P signalling causes sustained attenuation of protein synthesis, leading to memory impairment and neuronal loss. Re-establishing translation rates by inhibition of eIF2α-P activity, genetically or pharmacologically, restores memory and prevents neurodegeneration and extends survival. However, the experimental compounds used preclinically are unsuitable for use in humans, due to associated toxicity or poor pharmacokinetic properties. To discover compounds that have anti-eIF2α-P activity suitable for clinical use, we performed phenotypic screens on a NINDS small molecule library of 1040 drugs. We identified two compounds, trazodone hydrochloride and dibenzoylmethane, which reversed eIF2α-P-mediated translational attenuation in vitro and in vivo. Both drugs were markedly neuroprotective in two mouse models of neurodegeneration, using clinically relevant doses over a prolonged period of time, without systemic toxicity. Thus, in prion-diseased mice, both trazodone and dibenzoylmethane treatment restored memory deficits, abrogated development of neurological signs, prevented neurodegeneration and significantly prolonged survival. In tauopathy-frontotemporal dementia mice, both drugs were neuroprotective, rescued memory deficits and reduced hippocampal atrophy. Further, trazodone reduced p-tau burden. These compounds therefore represent potential new disease-modifying treatments for dementia. Trazodone in particular, a licensed drug, should now be tested in clinical trials in patients.
Keywords: dementia; drug repurposing; neurodegeneration; therapeutics.
© The Author (2017). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures
Comment in
-
Neurodegenerative disease: Halting neurodegeneration - are repurposed drugs the answer?Nat Rev Neurol. 2017 Jun;13(6):317. doi: 10.1038/nrneurol.2017.71. Epub 2017 May 5. Nat Rev Neurol. 2017. PMID: 28474684 No abstract available.
-
Drug repurposing to target proteostasis and prevent neurodegeneration: accelerating translational efforts.Brain. 2017 Jun 1;140(6):1544-1547. doi: 10.1093/brain/awx107. Brain. 2017. PMID: 28549133 No abstract available.
-
Reply: Trazodone to change the risk of neurodegeneration: bedside to bench.Brain. 2017 Aug 1;140(8):e48. doi: 10.1093/brain/awx150. Brain. 2017. PMID: 28641370 No abstract available.
-
Trazodone to change the risk of neurodegeneration: bedside to bench.Brain. 2017 Aug 1;140(8):e47. doi: 10.1093/brain/awx149. Brain. 2017. PMID: 28641375 No abstract available.
References
-
- Dinkova-Kostova AT, Talalay P. Relation of structure of curcumin analogs to their potencies as inducers of Phase 2 detoxification enzymes. Carcinogenesis 1999; 20: 911–14. - PubMed
-
- Frazier MC, Jackson KM, Jankowska-Stephens E, Anderson MG, Harris WB. Proteomic analysis of proteins altered by dibenzoylmethane in human prostatic cancer LNCaP cells. Proteomics 2004; 4: 2814–21. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

