Unmet needs in the first-line treatment of follicular lymphoma
- PMID: 28430865
- PMCID: PMC5834060
- DOI: 10.1093/annonc/mdx189
Unmet needs in the first-line treatment of follicular lymphoma
Abstract
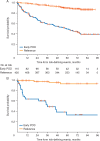
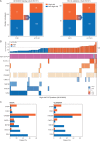
For the majority of patients with newly diagnosed follicular lymphoma (FL), current treatments, while not curative, allow for long remission durations. However, several important needs remain unaddressed. Studies have consistently shown that ∼20% of patients with FL experience disease progression within 2 years of first-line treatment, and consequently have a 50% risk of death in 5 years. Better characterization of this group of patients at diagnosis may provide insight into those in need of alternate or intensive therapies, facilitate a precision approach to inform clinical trials, and allow for improved patient counseling. Prognostic methods to date have employed clinical parameters, genomic methods, and a wide assortment of biological and biochemical markers, but none so far has been able to adequately identify this high-risk population. Advances in the first-line treatment of FL with chemoimmunotherapy have led to a median progression-free survival (PFS) of approximately 7 years; creating a challenge in the development of clinical trials where PFS is a primary end point. A surrogate end point that accurately predicts PFS would allow for new treatments to reach patients with FL sooner, or lessen toxicity, time, and expense to those patients requiring little to no therapy. Quality of response to treatment may predict PFS and overall survival in FL; as such complete response rates, either alone or in conjunction with PET imaging or minimal residual disease negativity, are being studied as surrogates, with complete response at 30 months after induction providing the strongest surrogacy evidence to date. A better understanding of how to optimize quality of life in the context of this chronic illness is another important focus deserving of further study. Ongoing efforts to address these important unmet needs are herein discussed.
Keywords: follicular lymphoma; indolent lymphoma; progression-free survival; surrogate end point.
© The Author 2017. Published by Oxford University Press on behalf of the European Society for Medical Oncology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
References
-
- A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. The Non-Hodgkin's Lymphoma Classification Project. Blood 1997; 89: 3909–3918. - PubMed
-
- Johnson PW, Rohatiner AZ, Whelan JS. et al. Patterns of survival in patients with recurrent follicular lymphoma: a 20-year study from a single center. J Clin Oncol 1995; 13: 140–147. - PubMed
-
- Swenson WT, Wooldridge JE, Lynch CF. et al. Improved survival of follicular lymphoma patients in the United States. J Clin Oncol 2005; 23: 5019–5026. - PubMed
-
- Salles G, Seymour JF, Offner F. et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet 2011; 377: 42–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

