IFT25, an intraflagellar transporter protein dispensable for ciliogenesis in somatic cells, is essential for sperm flagella formation
- PMID: 28430876
- PMCID: PMC6355109
- DOI: 10.1093/biolre/iox029
IFT25, an intraflagellar transporter protein dispensable for ciliogenesis in somatic cells, is essential for sperm flagella formation
Abstract
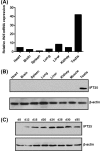
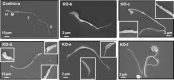
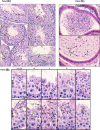
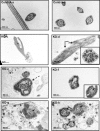
Intraflagellar transport (IFT) is a conserved mechanism essential for the assembly and maintenance of most eukaryotic cilia and flagella. However, IFT25, a component of the IFT complex, is not required for the formation of cilia in somatic tissues. In mice, the gene is highly expressed in the testis, and its expression is upregulated during the final phase when sperm flagella are formed. To investigate the role of IFT25 in sperm flagella formation, the gene was specifically disrupted in male germ cells. All homozygous knockout mice survived to adulthood and did not show any gross abnormalities. However, all homozygous knockout males were completely infertile. Sperm numbers were reduced and these sperm were completely immotile. Multiple morphological abnormalities were observed in sperm, including round heads, short and bent tails, with some tails showing branched flagella and others with frequent abnormal thicknesses, as well as swollen tips of the tail. Transmission electron microscopy revealed that flagellar accessory structures, including the fibrous sheath and outer dense fibers, were disorganized, and most sperm had also lost the "9+2" microtubule structure. In the testis, IFT25 forms a complex with other IFT proteins. In Ift25 knockout testes, IFT27, an IFT25 binding partner, was missing, and IFT20 and IFT81 levels were also reduced. Our findings suggest that IFT25, although not necessary for the formation of cilia in somatic cells, is indispensable for sperm flagellum formation and male fertility in mice.
Keywords: ciliogenesis; flagellogenesis; germ cells; intraflagellar transport; spermiogenesis.
© The Authors 2017. Published by Oxford University Press on behalf of Society for the Study of Reproduction. All rights reserved. For permissions, please journals.permissions@oup.com.
Figures


Similar articles
-
Intraflagellar transporter protein (IFT27), an IFT25 binding partner, is essential for male fertility and spermiogenesis in mice.Dev Biol. 2017 Dec 1;432(1):125-139. doi: 10.1016/j.ydbio.2017.09.023. Epub 2017 Sep 28. Dev Biol. 2017. PMID: 28964737 Free PMC article.
-
The essential role of intraflagellar transport protein IFT81 in male mice spermiogenesis and fertility.Am J Physiol Cell Physiol. 2020 Jun 1;318(6):C1092-C1106. doi: 10.1152/ajpcell.00450.2019. Epub 2020 Apr 1. Am J Physiol Cell Physiol. 2020. PMID: 32233951 Free PMC article.
-
Intraflagellar transport protein 74 is essential for spermatogenesis and male fertility in mice†.Biol Reprod. 2019 Jul 1;101(1):188-199. doi: 10.1093/biolre/ioz071. Biol Reprod. 2019. PMID: 31004481 Free PMC article.
-
Intraflagellar Transport (IFT) and Sperm Formation.Adv Exp Med Biol. 2025;1469:395-409. doi: 10.1007/978-3-031-82990-1_17. Adv Exp Med Biol. 2025. PMID: 40301266 Review.
-
The axoneme: the propulsive engine of spermatozoa and cilia and associated ciliopathies leading to infertility.J Assist Reprod Genet. 2016 Feb;33(2):141-56. doi: 10.1007/s10815-016-0652-1. Epub 2016 Jan 29. J Assist Reprod Genet. 2016. PMID: 26825807 Free PMC article. Review.
Cited by
-
COP9 signalosome complex subunit 5, an IFT20 binding partner, is essential to maintain male germ cell survival and acrosome biogenesis†.Biol Reprod. 2020 Feb 12;102(1):233-247. doi: 10.1093/biolre/ioz154. Biol Reprod. 2020. PMID: 31373619 Free PMC article.
-
The roles of intraflagellar transport (IFT) protein 25 in mammalian signaling transduction and flagellogenesis.Asian J Androl. 2022 May-Jun;24(3):238-242. doi: 10.4103/aja202179. Asian J Androl. 2022. PMID: 34747727 Free PMC article. Review.
-
Murine germ cell-specific disruption of Ift172 causes defects in spermiogenesis and male fertility.Reproduction. 2020 Apr;159(4):409-421. doi: 10.1530/REP-17-0789. Reproduction. 2020. PMID: 31958312 Free PMC article.
-
Rab GTPases in cilium formation and function.Small GTPases. 2018 Mar 4;9(1-2):76-94. doi: 10.1080/21541248.2017.1353847. Epub 2017 Oct 26. Small GTPases. 2018. PMID: 29072526 Free PMC article. Review.
-
ARL13B-Cerulean rescues Arl13b-null mouse from embryonic lethality and reveals a role for ARL13B in spermatogenesis.bioRxiv [Preprint]. 2025 Mar 26:2025.03.24.644968. doi: 10.1101/2025.03.24.644968. bioRxiv. 2025. PMID: 40196635 Free PMC article. Preprint.
References
-
- Pedersen LB, Mogensen JB, Christensen ST. Endocytic control of cellular signaling at the primary cilium. Trends Biochem Sci 2016; 41:784–797. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

