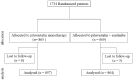
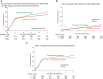
Low-density lipoprotein cholesterol targeting with pitavastatin + ezetimibe for patients with acute coronary syndrome and dyslipidaemia: the HIJ-PROPER study, a prospective, open-label, randomized trial
- PMID: 28430910
- PMCID: PMC5837267
- DOI: 10.1093/eurheartj/ehx162
Low-density lipoprotein cholesterol targeting with pitavastatin + ezetimibe for patients with acute coronary syndrome and dyslipidaemia: the HIJ-PROPER study, a prospective, open-label, randomized trial
Abstract
Aims: To elucidate the effects of intensive LDL-C lowering treatment with a standard dose of statin and ezetimibe in patients with dyslipidaemia and high risk of coronary events, targeting LDL-C less than 70 mg/dL (1.8 mmol/L), compared with standard LDL-C lowering lipid monotherapy targeting less than 100 mg/dL (2.6 mmol/L).
Methods and results: The HIJ-PROPER study is a prospective, randomized, open-label trial to assess whether intensive LDL-C lowering with standard-dose pitavastatin plus ezetimibe reduces cardiovascular events more than standard LDL-C lowering with pitavastatin monotherapy in patients with acute coronary syndrome (ACS) and dyslipidaemia. Patients were randomized to intensive lowering (target LDL-C < 70 mg/dL [1.8 mmol/L]; pitavastatin plus ezetimibe) or standard lowering (target LDL-C 90 mg/dL to 100 mg/dL [2.3-2.6 mmol/L]; pitavastatin monotherapy). The primary endpoint was a composite of all-cause death, non-fatal myocardial infarction, non-fatal stroke, unstable angina, and ischaemia-driven revascularization. Between January 2010 and April 2013, 1734 patients were enroled at 19 hospitals in Japan. Patients were followed for at least 36 months. Median follow-up was 3.86 years. Mean follow-up LDL-C was 65.1 mg/dL (1.68 mmol/L) for pitavastatin plus ezetimibe and 84.6 mg/dL (2.19 mmol/L) for pitavastatin monotherapy. LDL-C lowering with statin plus ezetimibe did not reduce primary endpoint occurrence in comparison with standard statin monotherapy (283/864, 32.8% vs. 316/857, 36.9%; HR 0.89, 95% CI 0.76-1.04, P = 0.152). In, ACS patients with higher cholesterol absorption, represented by elevated pre-treatment sitosterol, was associated with significantly lower incidence of the primary endpoint in the statin plus ezetimibe group (HR 0.71, 95% CI 0.56-0.91).
Conclusion: Although intensive lowering with standard pitavastatin plus ezetimibe showed no more cardiovascular benefit than standard pitavastatin monotherapy in ACS patients with dyslipidaemia, statin plus ezetimibe may be more effective than statin monotherapy in patients with higher cholesterol absorption; further confirmation is needed.
Trial no: UMIN000002742, registered as an International Standard Randomized Controlled Trial.
Keywords: Acute coronary syndrome; Clinical trial; Combination therapy; Ezetimibe; Lipid-lowering; Pitavastatin.
© The Author 2017. Published by Oxford University Press on behalf of the European Society of Cardiology
Figures
Comment in
-
Reduction of LDL-C-related residual cardiovascular risk with ezetimibe: are mechanistic considerations warranted in practice?Eur Heart J. 2017 Aug 1;38(29):2276-2278. doi: 10.1093/eurheartj/ehx275. Eur Heart J. 2017. PMID: 28525583 No abstract available.
Similar articles
-
Baseline low-density lipoprotein cholesterol predicts the benefit of adding ezetimibe on statin in statin-naïve acute coronary syndrome.Sci Rep. 2021 Apr 5;11(1):7480. doi: 10.1038/s41598-021-87098-x. Sci Rep. 2021. PMID: 33820931 Free PMC article. Clinical Trial.
-
Rationale, design features, and baseline characteristics: The Heart Institute of Japan-PRoper level of lipid lOwering with Pitavastatin and Ezetimibe in acute coRonary syndrome (HIJ-PROPER).J Cardiol. 2017 Mar;69(3):536-541. doi: 10.1016/j.jjcc.2016.05.002. Epub 2016 Jun 24. J Cardiol. 2017. PMID: 27349705 Clinical Trial.
-
Baseline serum sitosterol level as predictor of adverse clinical events in acute coronary syndrome patients with dyslipidaemia: A sub-analysis of HIJ-PROPER.Atherosclerosis. 2018 Jul;274:139-145. doi: 10.1016/j.atherosclerosis.2018.04.036. Epub 2018 May 5. Atherosclerosis. 2018. PMID: 29772482
-
The clinical effectiveness and safety of low/moderate-intensity statins & ezetimibe combination therapy vs. high-intensity statin monotherapy: a systematic review and meta-analysis.BMC Cardiovasc Disord. 2024 Nov 20;24(1):660. doi: 10.1186/s12872-024-04144-y. BMC Cardiovasc Disord. 2024. PMID: 39567875 Free PMC article.
-
Impact of early initiation of ezetimibe in patients with acute coronary syndrome: A systematic review and meta-analysis.Eur J Intern Med. 2024 Jun;124:99-107. doi: 10.1016/j.ejim.2024.02.004. Epub 2024 Feb 9. Eur J Intern Med. 2024. PMID: 38336550
Cited by
-
Personalized management of dyslipidemias in patients with diabetes-it is time for a new approach (2022).Cardiovasc Diabetol. 2022 Nov 28;21(1):263. doi: 10.1186/s12933-022-01684-5. Cardiovasc Diabetol. 2022. PMID: 36443827 Free PMC article. Review.
-
Baseline low-density lipoprotein cholesterol predicts the benefit of adding ezetimibe on statin in statin-naïve acute coronary syndrome.Sci Rep. 2021 Apr 5;11(1):7480. doi: 10.1038/s41598-021-87098-x. Sci Rep. 2021. PMID: 33820931 Free PMC article. Clinical Trial.
-
Dyslipidemia: A Narrative Review on Pharmacotherapy.Pharmaceuticals (Basel). 2024 Feb 23;17(3):289. doi: 10.3390/ph17030289. Pharmaceuticals (Basel). 2024. PMID: 38543075 Free PMC article. Review.
-
Polyunsaturated Fatty Acid Impact on Clinical Outcomes in Acute Coronary Syndrome Patients With Dyslipidemia: Subanalysis of HIJ-PROPER.J Am Heart Assoc. 2019 Aug 20;8(16):e012953. doi: 10.1161/JAHA.119.012953. Epub 2019 Aug 8. J Am Heart Assoc. 2019. PMID: 31390907 Free PMC article. Clinical Trial.
-
Effects of Non-statin Lipid-Modifying Agents on Cardiovascular Morbidity and Mortality Among Statin-Treated Patients: A Systematic Review and Network Meta-Analysis.Front Pharmacol. 2019 May 22;10:547. doi: 10.3389/fphar.2019.00547. eCollection 2019. Front Pharmacol. 2019. PMID: 31191304 Free PMC article.
References
-
- Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383–1389. - PubMed
-
- Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R.. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366:1267–1278. - PubMed
-
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA 2001;285:2486–2497. - PubMed
-
- Brunzell JD, Davidson M, Furberg CD, Goldberg RB, Howard BV, Stein JH, Witztum JL.. Lipoprotein management in patients with cardiometabolic risk: consensus statement from the American Diabetes Association and the American College of Cardiology Foundation. Diabetes Care 2008;31:811–822. - PubMed
-
- Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM.. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495–1504. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous