Biological functions of fucose in mammals
- PMID: 28430973
- PMCID: PMC5458543
- DOI: 10.1093/glycob/cwx034
Biological functions of fucose in mammals
Abstract
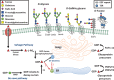
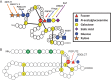
Fucose is a 6-deoxy hexose in the l-configuration found in a large variety of different organisms. In mammals, fucose is incorporated into N-glycans, O-glycans and glycolipids by 13 fucosyltransferases, all of which utilize the nucleotide-charged form, GDP-fucose, to modify targets. Three of the fucosyltransferases, FUT8, FUT12/POFUT1 and FUT13/POFUT2, are essential for proper development in mice. Fucose modifications have also been implicated in many other biological functions including immunity and cancer. Congenital mutations of a Golgi apparatus localized GDP-fucose transporter causes leukocyte adhesion deficiency type II, which results in severe developmental and immune deficiencies, highlighting the important role fucose plays in these processes. Additionally, changes in levels of fucosylated proteins have proven as useful tools for determining cancer diagnosis and prognosis. Chemically modified fucose analogs can be used to alter many of these fucose dependent processes or as tools to better understand them. In this review, we summarize the known roles of fucose in mammalian physiology and pathophysiology. Additionally, we discuss recent therapeutic advances for cancer and other diseases that are a direct result of our improved understanding of the role that fucose plays in these systems.
Keywords: cancer; development; fucose; fucosyltransferase; immunology.
© The Author 2017. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- Adamczyk B, Tharmalingam T, Rudd PM. 2012. Glycans as cancer biomarkers. Biochim Biophys Acta (BBA)-Gen Subjects. 1820:1347–1353. - PubMed
-
- Al-Shareffi E, Chaubard JL, Leonhard-Melief C, Wang SK, Wong CH, Haltiwanger RS. 2013. 6-Alkynyl fucose is a bioorthogonal analog for O-fucosylation of epidermal growth factor-like repeats and thrombospondin type-1 repeats by protein O-fucosyltransferases 1 and 2. Glycobiology. 23:188–198. - PMC - PubMed
-
- Aldahmesh MA, Alshammari MJ, Khan AO, Mohamed JY, Alhabib FA, Alkuraya FS. 2013. The syndrome of microcornea, myopic chorioretinal atrophy, and telecanthus (MMCAT) is caused by mutations in ADAMTS18. Hum Mutat. 34:1195–1199. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

