Global Impact of Rotavirus Vaccination on Childhood Hospitalizations and Mortality From Diarrhea
- PMID: 28430997
- PMCID: PMC5543929
- DOI: 10.1093/infdis/jix186
Global Impact of Rotavirus Vaccination on Childhood Hospitalizations and Mortality From Diarrhea
Abstract
In 2006, 2 rotavirus vaccines were licensed. We summarize the impact of rotavirus vaccination on hospitalizations and deaths from rotavirus and all-cause acute gastroenteritis (AGE) during the first 10 years since vaccine licensure, including recent evidence from countries with high child mortality. We used standardized guidelines (PRISMA) to identify observational evaluations of rotavirus vaccine impact among children <5 years of age that presented at least 12 months of pre- and post-vaccine introduction surveillance data. We identified 57 articles from 27 countries. Among children <5 years of age, the median percentage reduction in AGE hospitalizations was 38% overall and 41%, 30%, and 46% in countries with low, medium, and high child mortality, respectively. Hospitalizations and emergency department visits due to rotavirus AGE were reduced by a median of 67% overall and 71%, 59%, and 60% in countries with low, medium, and high child mortality, respectively. Implementation of rotavirus vaccines has substantially decreased hospitalizations from rotavirus and all-cause AGE.
Keywords: Rotavirus vaccine; diarrhea; rotavirus.
Published by Oxford University Press for the Infectious Diseases Society of America 2017. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Conflict of interest statement
The authors either do not have a commercial or other association that might pose a conflict of interest;
Figures

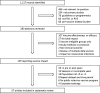



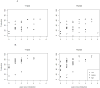

Comment in
-
Vaccine Impact Data Should Support Country Decision Making.J Infect Dis. 2017 Jun 1;215(11):1634-1636. doi: 10.1093/infdis/jix187. J Infect Dis. 2017. PMID: 28431126 Free PMC article. No abstract available.
References
-
- Centers for Disease C, Prevention. Rotavirus surveillance--worldwide, 2001–2008. MMWR Morb Mortal Wkly Rep. 2008;57(46):1255–7. - PubMed
-
- Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354(1):11–22. - PubMed
-
- Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354(1):23–33. - PubMed
-
- Organization WH. Rotavirus vaccines: WHO position paper- January 2013. Weekly epidemiological review. 2013;88(5):49–64.
-
- Tate JE, Cortese MM, Payne DC, et al. Uptake, impact, and effectiveness of rotavirus vaccination in the United States: review of the first 3 years of postlicensure data. Pediatr Infect Dis J. 2011;30(1 Suppl):S56–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

