BRG1 interacts with SOX10 to establish the melanocyte lineage and to promote differentiation
- PMID: 28431046
- PMCID: PMC5499657
- DOI: 10.1093/nar/gkx259
BRG1 interacts with SOX10 to establish the melanocyte lineage and to promote differentiation
Abstract
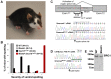
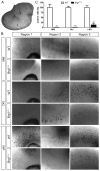
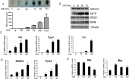
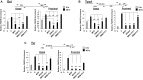
Mutations in SOX10 cause neurocristopathies which display varying degrees of hypopigmentation. Using a sensitized mutagenesis screen, we identified Smarca4 as a modifier gene that exacerbates the phenotypic severity of Sox10 haplo-insufficient mice. Conditional deletion of Smarca4 in SOX10 expressing cells resulted in reduced numbers of cranial and ventral trunk melanoblasts. To define the requirement for the Smarca4 -encoded BRG1 subunit of the SWI/SNF chromatin remodeling complex, we employed in vitro models of melanocyte differentiation in which induction of melanocyte-specific gene expression is closely linked to chromatin alterations. We found that BRG1 was required for expression of Dct, Tyrp1 and Tyr, genes that are regulated by SOX10 and MITF and for chromatin remodeling at distal and proximal regulatory sites. SOX10 was found to physically interact with BRG1 in differentiating melanocytes and binding of SOX10 to the Tyrp1 distal enhancer temporally coincided with recruitment of BRG1. Our data show that SOX10 cooperates with MITF to facilitate BRG1 binding to distal enhancers of melanocyte-specific genes. Thus, BRG1 is a SOX10 co-activator, required to establish the melanocyte lineage and promote expression of genes important for melanocyte function.
© The Author(s) 2017. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





Similar articles
-
BAF60A mediates interactions between the microphthalmia-associated transcription factor and the BRG1-containing SWI/SNF complex during melanocyte differentiation.J Cell Physiol. 2019 Jul;234(7):11780-11791. doi: 10.1002/jcp.27840. Epub 2018 Dec 4. J Cell Physiol. 2019. PMID: 30515787 Free PMC article.
-
SWI/SNF enzymes promote SOX10- mediated activation of myelin gene expression.PLoS One. 2013 Jul 16;8(7):e69037. doi: 10.1371/journal.pone.0069037. Print 2013. PLoS One. 2013. PMID: 23874858 Free PMC article.
-
Transcription factor MITF and remodeller BRG1 define chromatin organisation at regulatory elements in melanoma cells.Elife. 2015 Mar 24;4:e06857. doi: 10.7554/eLife.06857. Elife. 2015. PMID: 25803486 Free PMC article.
-
A sensitized mutagenesis screen identifies Gli3 as a modifier of Sox10 neurocristopathy.Hum Mol Genet. 2008 Jul 15;17(14):2118-31. doi: 10.1093/hmg/ddn110. Epub 2008 Apr 7. Hum Mol Genet. 2008. PMID: 18397875 Free PMC article.
-
The importance of having your SOX on: role of SOX10 in the development of neural crest-derived melanocytes and glia.Oncogene. 2003 May 19;22(20):3024-34. doi: 10.1038/sj.onc.1206442. Oncogene. 2003. PMID: 12789277 Review.
Cited by
-
CEACAM1 is a direct SOX10 target and inhibits melanoma immune infiltration and stemness.iScience. 2022 Nov 7;25(12):105524. doi: 10.1016/j.isci.2022.105524. eCollection 2022 Dec 22. iScience. 2022. PMID: 36437876 Free PMC article.
-
Sperm-Specific Glycolysis Enzyme Glyceraldehyde-3-Phosphate Dehydrogenase Regulated by Transcription Factor SOX10 to Promote Uveal Melanoma Tumorigenesis.Front Cell Dev Biol. 2021 Jun 25;9:610683. doi: 10.3389/fcell.2021.610683. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34249897 Free PMC article.
-
Epigenetic and pharmacological control of pigmentation via Bromodomain Protein 9 (BRD9).Pigment Cell Melanoma Res. 2023 Jan;36(1):19-32. doi: 10.1111/pcmr.13068. Epub 2022 Oct 3. Pigment Cell Melanoma Res. 2023. PMID: 36112085 Free PMC article.
-
BAF60A mediates interactions between the microphthalmia-associated transcription factor and the BRG1-containing SWI/SNF complex during melanocyte differentiation.J Cell Physiol. 2019 Jul;234(7):11780-11791. doi: 10.1002/jcp.27840. Epub 2018 Dec 4. J Cell Physiol. 2019. PMID: 30515787 Free PMC article.
-
Neural crest contributions to the ear: Implications for congenital hearing disorders.Hear Res. 2019 May;376:22-32. doi: 10.1016/j.heares.2018.11.005. Epub 2018 Nov 14. Hear Res. 2019. PMID: 30455064 Free PMC article. Review.
References
-
- Bolande R.P. Neurocristopathy: its growth and development in 20 years. Pediatr. Pathol. Lab. Med. 1997; 17:1–25. - PubMed
-
- Pingault V., Bondurand N., Kuhlbrodt K., Goerich D.E., Prehu M.O., Puliti A., Herbarth B., Hermans-Borgmeyer I., Legius E., Matthijs G. et al. . SOX10 mutations in patients with Waardenburg-Hirschsprung disease. Nat. Genet. 1998; 18:171–173. - PubMed
-
- Baldwin C.T., Hoth C.F., Amos J.A., da-Silva E.O., Milunsky A.. An exonic mutation in the HuP2 paired domain gene causes Waardenburg's syndrome. Nature. 1992; 355:637–638. - PubMed
-
- Tassabehji M., Read A.P., Newton V.E., Harris R., Balling R., Gruss P., Strachan T.. Waardenburg's syndrome patients have mutations in the human homologue of the Pax-3 paired box gene. Nature. 1992; 355:635–636. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

