Respiratory chain enzyme deficiency induces mitochondrial location of actin-binding gelsolin to modulate the oligomerization of VDAC complexes and cell survival
- PMID: 28431142
- PMCID: PMC6192415
- DOI: 10.1093/hmg/ddx144
Respiratory chain enzyme deficiency induces mitochondrial location of actin-binding gelsolin to modulate the oligomerization of VDAC complexes and cell survival
Abstract
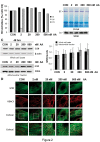
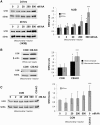
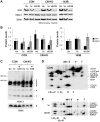
Despite considerable knowledge on the genetic basis of mitochondrial disorders, their pathophysiological consequences remain poorly understood. We previously used two-dimensional difference gel electrophoresis analyses to define a protein profile characteristic for respiratory chain complex III-deficiency that included a significant overexpression of cytosolic gelsolin (GSN), a cytoskeletal protein that regulates the severing and capping of the actin filaments. Biochemical and immunofluorescence assays confirmed a specific increase of GSN levels in the mitochondria from patients' fibroblasts and from transmitochondrial cybrids with complex III assembly defects. A similar effect was obtained in control cells upon treatment with antimycin A in a dose-dependent manner, showing that the enzymatic inhibition of complex III is sufficient to promote the mitochondrial localization of GSN. Mitochondrial subfractionation showed the localization of GSN to the mitochondrial outer membrane, where it interacts with the voltage-dependent anion channel protein 1 (VDAC1). In control cells, VDAC1 was present in five stable oligomeric complexes, which showed increased levels and a modified distribution pattern in the complex III-deficient cybrids. Downregulation of GSN expression induced cell death in both cell types, in parallel with the specific accumulation of VDAC1 dimers and the release of mitochondrial cytochrome c into the cytosol, indicating a role for GSN in the oligomerization of VDAC complexes and in the prevention of apoptosis. Our results demonstrate that respiratory chain complex III dysfunction induces the physiological upregulation and mitochondrial location of GSN, probably to promote cell survival responses through the modulation of the oligomeric state of the VDAC complexes.
© The Author 2017. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures




Similar articles
-
Altered Expression Ratio of Actin-Binding Gelsolin Isoforms Is a Novel Hallmark of Mitochondrial OXPHOS Dysfunction.Cells. 2020 Aug 19;9(9):1922. doi: 10.3390/cells9091922. Cells. 2020. PMID: 32824961 Free PMC article.
-
The mitochondrial voltage-dependent anion channel 1 in tumor cells.Biochim Biophys Acta. 2015 Oct;1848(10 Pt B):2547-75. doi: 10.1016/j.bbamem.2014.10.040. Epub 2014 Nov 4. Biochim Biophys Acta. 2015. PMID: 25448878 Review.
-
Villin-1 and Gelsolin Regulate Changes in Actin Dynamics That Affect Cell Survival Signaling Pathways and Intestinal Inflammation.Gastroenterology. 2018 Apr;154(5):1405-1420.e2. doi: 10.1053/j.gastro.2017.12.016. Epub 2017 Dec 21. Gastroenterology. 2018. PMID: 29274870 Free PMC article.
-
Hypoxia Augments Increased HIF-1α and Reduced Survival Protein p-Akt in Gelsolin (GSN)-Dependent Cardiomyoblast Cell Apoptosis.Cell Biochem Biophys. 2016 Jun;74(2):221-8. doi: 10.1007/s12013-016-0729-6. Epub 2016 May 18. Cell Biochem Biophys. 2016. PMID: 27193608
-
Mitochondrial VDAC, the Na+/Ca2+ Exchanger, and the Ca2+ Uniporter in Ca2+ Dynamics and Signaling.Adv Exp Med Biol. 2017;981:323-347. doi: 10.1007/978-3-319-55858-5_13. Adv Exp Med Biol. 2017. PMID: 29594867 Review.
Cited by
-
Altered Expression Ratio of Actin-Binding Gelsolin Isoforms Is a Novel Hallmark of Mitochondrial OXPHOS Dysfunction.Cells. 2020 Aug 19;9(9):1922. doi: 10.3390/cells9091922. Cells. 2020. PMID: 32824961 Free PMC article.
-
Plasma Gelsolin Reinforces the Diagnostic Value of FGF-21 and GDF-15 for Mitochondrial Disorders.Int J Mol Sci. 2021 Jun 15;22(12):6396. doi: 10.3390/ijms22126396. Int J Mol Sci. 2021. PMID: 34203775 Free PMC article.
-
miR-19-3p/GRSF1/COX1 axis attenuates early brain injury via maintaining mitochondrial function after subarachnoid haemorrhage.Stroke Vasc Neurol. 2025 Jun 30;10(3):297-310. doi: 10.1136/svn-2024-003099. Stroke Vasc Neurol. 2025. PMID: 39266212 Free PMC article.
-
Natural Antioxidant By-Product Mixture Counteracts the Effects of Aflatoxin B1 and Ochratoxin A Exposure of Piglets after Weaning: A Proteomic Survey on Liver Microsomal Fraction.Toxins (Basel). 2023 Apr 19;15(4):299. doi: 10.3390/toxins15040299. Toxins (Basel). 2023. PMID: 37104237 Free PMC article.
-
Response to chronic sustained hypoxia: increased cytosolic gelsolin and decreased plasma gelsolin levels.J Mol Histol. 2024 Oct;55(5):1009-1019. doi: 10.1007/s10735-024-10248-8. Epub 2024 Aug 22. J Mol Histol. 2024. PMID: 39172327
References
-
- Iwata S., Lee J.W., Okada K., Lee J.K., Iwata M., Rasmussen B., Link T.A., Ramaswamy S., Jap B.K. (1998) Complete structure of the 11-subunit bovine mitochondrial cytochrome bc1 complex. Science, 281, 64–71. - PubMed
-
- Xia D., Yu C.A., Kim H., Xia J.Z., Kachurin A.M., Zhang L., Yu L., Deisenhofer J. (1997) Crystal structure of the cytochrome bc1 complex from bovine heart mitochondria. Science, 277, 60–66. - PubMed
-
- Bénit P., Lebon S., Rustin P. (2009) Respiratory-chain diseases related to complex III deficiency. Biochim. Biophys. Acta, 1793, 181–185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

