Engineering a high-affinity peptide binding site into the anti-CEA mAb M5A
- PMID: 28431161
- PMCID: PMC5914451
- DOI: 10.1093/protein/gzx016
Engineering a high-affinity peptide binding site into the anti-CEA mAb M5A
Abstract
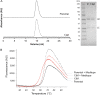
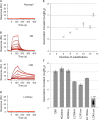
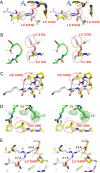
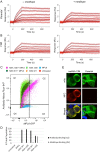
We have previously identified a cyclic peptide called meditope which binds to the central cavity of the Fab portion of cetuximab and shown that this peptide binding site can be grafted, or 'meditope-enabled', onto trastuzumab. This peptide has been shown to act as a hitch for the non-covalent attachment of imaging agents to meditope-enabled antibodies. Herein, we explore the process of grafting this peptide binding site onto M5A, an anti-CEA antibody in clinical trials for cancer diagnostics. In order to explore the contributions of the amino acids, we sequentially introduced pairs of amino acid substitutions into the Fab and then we reverse-substituted key residues in the presence of the other substitutions. We demonstrate that Pro40Thr, Gly41Asn, Phe83Ile and Thr85Asp in the light chain are sufficient to recreate the meditope binding site in M5A with single-digit micromolar affinity. We show that Pro40 abrogates peptide binding in the presence of the other 12 residue substitutions, and that the presence of all 13 substitutions does not interfere with antibody:antigen recognition. Collectively, these studies provide detailed insight for defining and fine-tuning the binding affinity of the meditope binding site within an antibody.
Keywords: CEA; antibody engineering; antibody-drug conjugate; grafting; meditope.
© The Author 2017. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures

Similar articles
-
Refining the Quality of Monoclonal Antibodies: Grafting Unique Peptide-Binding Site in the Fab Framework.Methods Mol Biol. 2019;1904:293-298. doi: 10.1007/978-1-4939-8958-4_12. Methods Mol Biol. 2019. PMID: 30539475 Free PMC article.
-
Identification and grafting of a unique peptide-binding site in the Fab framework of monoclonal antibodies.Proc Natl Acad Sci U S A. 2013 Oct 22;110(43):17456-61. doi: 10.1073/pnas.1307309110. Epub 2013 Oct 7. Proc Natl Acad Sci U S A. 2013. PMID: 24101516 Free PMC article.
-
Natural and non-natural amino-acid side-chain substitutions: affinity and diffraction studies of meditope-Fab complexes.Acta Crystallogr F Struct Biol Commun. 2016 Nov 1;72(Pt 11):820-830. doi: 10.1107/S2053230X16016149. Epub 2016 Oct 24. Acta Crystallogr F Struct Biol Commun. 2016. PMID: 27834791 Free PMC article.
-
Quantifying antibody binding: techniques and therapeutic implications.MAbs. 2025 Dec;17(1):2459795. doi: 10.1080/19420862.2025.2459795. Epub 2025 Feb 16. MAbs. 2025. PMID: 39957177 Free PMC article. Review.
-
Protein Grafting Techniques: From Peptide Epitopes to Lasso-Grafted Neobiologics.Chempluschem. 2024 Aug;89(8):e202400152. doi: 10.1002/cplu.202400152. Epub 2024 May 27. Chempluschem. 2024. PMID: 38693599 Review.
Cited by
-
A Platform To Enhance Quantitative Single Molecule Localization Microscopy.J Am Chem Soc. 2018 Oct 10;140(40):12785-12797. doi: 10.1021/jacs.8b04939. Epub 2018 Sep 26. J Am Chem Soc. 2018. PMID: 30256630 Free PMC article.
-
Engineered coiled-coil HIF1α protein domain mimic.Biomater Sci. 2024 May 28;12(11):2951-2959. doi: 10.1039/d4bm00354c. Biomater Sci. 2024. PMID: 38656316 Free PMC article.
-
Template-Catalyzed, Disulfide Conjugation of Monoclonal Antibodies Using a Natural Amino Acid Tag.Bioconjug Chem. 2018 Jun 20;29(6):2074-2081. doi: 10.1021/acs.bioconjchem.8b00284. Epub 2018 May 25. Bioconjug Chem. 2018. PMID: 29763554 Free PMC article.
-
Mechanically interlocked functionalization of monoclonal antibodies.Nat Commun. 2018 Apr 20;9(1):1580. doi: 10.1038/s41467-018-03976-5. Nat Commun. 2018. PMID: 29679060 Free PMC article.
-
Refining the Quality of Monoclonal Antibodies: Grafting Unique Peptide-Binding Site in the Fab Framework.Methods Mol Biol. 2019;1904:293-298. doi: 10.1007/978-1-4939-8958-4_12. Methods Mol Biol. 2019. PMID: 30539475 Free PMC article.
References
-
- Avery K.N., Zer C., Bzymek K.P. and Williams J.C. (2015) Sci. Rep., 5, 7817 doi:10.1038/srep07817. - DOI - PMC - PubMed
-
- Behrens C.R. and Liu B. (2014) mAbs, 6, 46–53. doi:10.4161/mabs.26632. - DOI - PMC - PubMed
-
- Donaldson J.M., Zer C., Avery K.N., Bzymek K.P., Horne D.A. and Williams J.C. (2013) Proc. Natl Acad. Sci. U.S.A., 110, 17456–17461. doi:10.1073/pnas.1307309110. - DOI - PMC - PubMed
-
- Jain M., Kamal N. and Batra S.K. (2007) Trends Biotechnol., 25, 307–316. doi:10.1016/j.tibtech.2007.05.001. - DOI - PubMed
-
- Jakob C.G., Edalji R., Judge R.A., DiGiammarino E., Li Y., Gu J. and Ghayur T. (2013) mAbs, 5, 358–363. doi:10.4161/mabs.23977. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

