Spontaneous Chitin Accumulation in Airways and Age-Related Fibrotic Lung Disease
- PMID: 28431248
- PMCID: PMC5444468
- DOI: 10.1016/j.cell.2017.03.044
Spontaneous Chitin Accumulation in Airways and Age-Related Fibrotic Lung Disease
Abstract
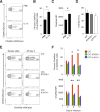
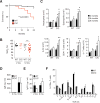
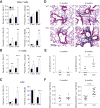
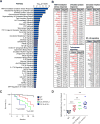
The environmentally widespread polysaccharide chitin is degraded and recycled by ubiquitous bacterial and fungal chitinases. Although vertebrates express active chitinases from evolutionarily conserved loci, their role in mammalian physiology is unclear. We show that distinct lung epithelial cells secrete acidic mammalian chitinase (AMCase), which is required for airway chitinase activity. AMCase-deficient mice exhibit premature morbidity and mortality, concomitant with accumulation of environmentally derived chitin polymers in the airways and expression of pro-fibrotic cytokines. Over time, these mice develop spontaneous pulmonary fibrosis, which is ameliorated by restoration of lung chitinase activity by genetic or therapeutic approaches. AMCase-deficient epithelial cells express fibrosis-associated gene sets linked with cell stress pathways. Mice with lung fibrosis due to telomere dysfunction and humans with interstitial lung disease also accumulate excess chitin polymers in their airways. These data suggest that altered chitin clearance could exacerbate fibrogenic pathways in the setting of lung diseases characterized by epithelial cell dysfunction.
Keywords: AMCase; age-related disease; chitin; chitinase; epithelium; interleukins; interstitial lung disease; polysaccharide; pulmonary fibrosis.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures



References
-
- Adrangi S, Faramarzi MA. From bacteria to human: a journey into the world of chitinases. Biotechnol Adv. 2008;31:1786–1795. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

