Strategies for monitoring and combating resistance to combination kinase inhibitors for cancer therapy
- PMID: 28431544
- PMCID: PMC5399860
- DOI: 10.1186/s13073-017-0431-3
Strategies for monitoring and combating resistance to combination kinase inhibitors for cancer therapy
Abstract
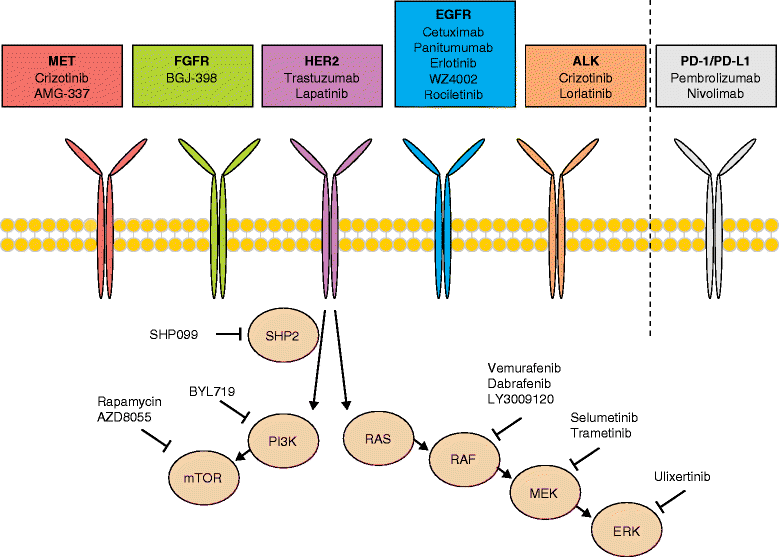
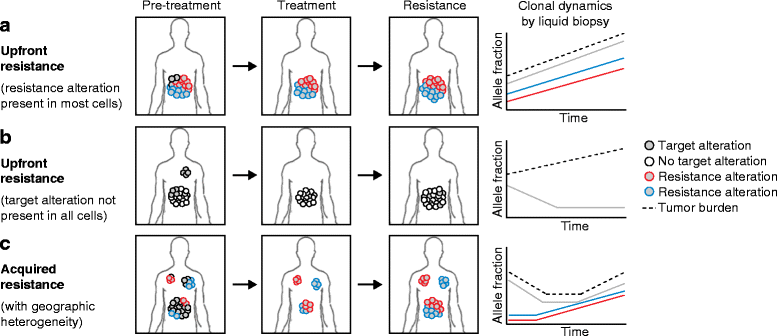
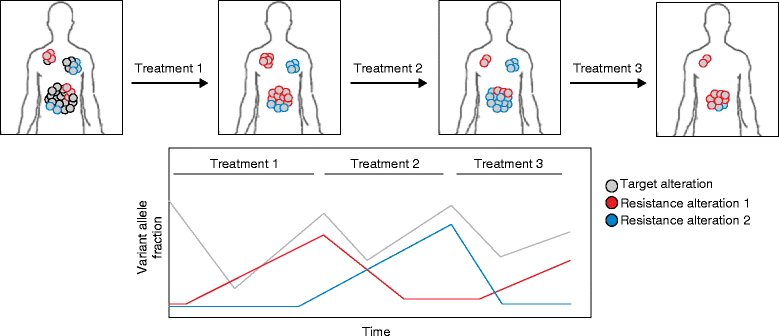
Targeted therapies such as kinase inhibitors and monoclonal antibodies have dramatically altered cancer care in recent decades. Although these targeted therapies have improved patient outcomes in several cancer types, resistance ultimately develops to these agents. One potential strategy proposed to overcome acquired resistance involves taking repeat tumor biopsies at the time of disease progression, to identify the specific molecular mechanism driving resistance in an individual patient and to select a new agent or combination of agents capable of surmounting that specific resistance mechanism. However, recent studies sampling multiple metastatic lesions upon acquired resistance, or employing "liquid biopsy" analyses of circulating tumor DNA, have revealed that multiple, heterogeneous resistance mechanisms can emerge in distinct tumor subclones in the same patient. This heterogeneity represents a major clinical challenge for devising therapeutic strategies to overcome resistance. In many cancers, multiple drug resistance mechanisms often converge to reactivate the original pathway targeted by the drug. This convergent evolution creates an opportunity to target a common signaling node to overcome resistance. Furthermore, integration of liquid biopsy approaches into clinical practice may allow real-time monitoring of emerging resistance alterations, allowing intervention prior to standard detection of radiographic progression. In this review, we discuss recent advances in understanding tumor heterogeneity and resistance to targeted therapies, focusing on combination kinase inhibitors, and we discuss approaches to address these issues in the clinic.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

