Impact of study outcome on submission and acceptance metrics for peer reviewed medical journals: six year retrospective review of all completed GlaxoSmithKline human drug research studies
- PMID: 28432051
- PMCID: PMC5421444
- DOI: 10.1136/bmj.j1726
Impact of study outcome on submission and acceptance metrics for peer reviewed medical journals: six year retrospective review of all completed GlaxoSmithKline human drug research studies
Abstract
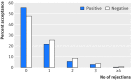
Objectives To determine whether the outcome of drug studies influenced submission and/or acceptance rates for publication in peer reviewed medical journals.Design A six year retrospective review of publication status by study outcome for all human drug research studies conducted by a single industry sponsor (GlaxoSmithKline) that completed from 1 January 2009 to 30 June 2014 and were therefore due for manuscript submission (per the sponsor's policy) to peer reviewed journals within 18 months of study completion-that is, 31 December 2015. In addition, manuscripts from studies completing after 30 June 2014 were included irrespective of outcome if they were submitted before 31 December 2015.Setting Studies conducted by a single industry sponsor (GlaxoSmithKline)Studies reviewed 1064 human drug research studies.Main outcome measures All studies were assigned a publication status at 26 February 2016 including (as applicable): study completion date, date of first primary manuscript submission, number of submissions, journal decision(s), and publication date. All studies were also classified with assessors blinded to publication status as "positive" (perceived favorable outcome for the drug under study), "negative" (perceived unfavorable outcome for the drug under study), mixed, or non-comparative based on the presence and outcome of the primary outcome measure(s) for each study. "Negative" studies included safety studies in which the primary outcome was achieved but was adverse for the drug under study. For the total cohort and each of the four study outcomes, measures included descriptive statistics for study phase, time from study completion to submission and publication, and number and outcome (accepted/rejected) of publication submissions.Results Of the 1064 studies (phase I-IV, interventional and non-interventional) included, 321 had study outcomes classified as positive, 155 as negative, 52 as mixed, and 536 as non-comparative. At the time of publication cut-off date (26 February 2016), 904 (85%) studies had been submitted for publication as full manuscripts and 751 (71%) had been successfully published or accepted, with 100 (9%) still under journal review. An additional 77 (7%) studies were conference abstracts and were not included in submission or publication rates. Submission rates by study outcome were 79% for the 321 studies with positive outcomes, 92% for the 155 with negative outcomes, 94% for the 52 with mixed outcomes, and 85% for the 536 non-comparative studies; while rates of publication at the cut-off date were 66%, 77%, 77%, and 71%, respectively. Median time from study completion to submission was 537 days (interquartile range 396-638 days) and 823 days (650-1063 days) from completion to publication, with similar times observed across study outcomes. First time acceptance rates were 56% for studies with positive outcomes and 48% for studies with negative outcomes. Over 10% of studies across all categories required three or more submissions to achieve successful publication. At the time of analysis, 83 studies had not been submitted for publication, including 49 bioequivalence studies with positive outcomes and 33 non-comparative studies. Most studies (98%, 1041/1064) had results posted to one or more public registers, including all studies subject to FDAAA (Food and Drug Administration Amendments Act) requirements for posting to www.clinicaltrials.govConclusions Over the period studied, there was no evidence of submission or publication bias: 92% of studies with negative outcomes were submitted for publication by the cut-off date versus 79% of those with positive outcomes. Publication rates were slightly higher for studies with a negative (that is, unfavorable) outcome compared with a positive outcome, despite a slightly lower rate of acceptance at first submission. Many studies required multiple submission attempts before they were accepted for publication. Analyses focusing solely on publication rates do not take into account unsuccessful efforts to publish. Sponsors and journal editors should share similar information to contribute to better understanding of issues and barriers to full transparency.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form. All four authors were employees and shareholders in GSK at the time this research was conducted. GSK sponsored the >1000 individual studies described in this analysis, paid the salaries of the four named authors, and funded the services acknowledged above, but was not otherwise involved in the project conception or execution. BM and BDeC are members of the Medical Publishing Insights and Practices Steering Committee, which is chaired by BM.
Figures
Comment in
-
Tackling submission and publication bias.BMJ. 2017 Jul 17;358:j3436. doi: 10.1136/bmj.j3436. BMJ. 2017. PMID: 28716968 No abstract available.
-
Authors' reply to Spiegel and colleagues.BMJ. 2017 Jul 18;358:j3444. doi: 10.1136/bmj.j3444. BMJ. 2017. PMID: 28720643 No abstract available.
Similar articles
-
Role of editorial and peer review processes in publication bias: analysis of drug trials submitted to eight medical journals.PLoS One. 2014 Aug 12;9(8):e104846. doi: 10.1371/journal.pone.0104846. eCollection 2014. PLoS One. 2014. PMID: 25118182 Free PMC article.
-
Journal response types and times: the outcomes of manuscripts finalised for submission by the University of the Free State School of Medicine medical editor, South Africa.Pan Afr Med J. 2020 Jul 24;36:212. doi: 10.11604/pamj.2020.36.212.24175. eCollection 2020. Pan Afr Med J. 2020. PMID: 32963678 Free PMC article.
-
Metrics for Original Research Articles in the AJR: From First Submission to Final Publication.AJR Am J Roentgenol. 2015 Jun;204(6):1152-6. doi: 10.2214/AJR.14.13944. AJR Am J Roentgenol. 2015. PMID: 26001223
-
Peer review comments on drug trials submitted to medical journals differ depending on sponsorship, results and acceptance: a retrospective cohort study.BMJ Open. 2015 Sep 30;5(9):e007961. doi: 10.1136/bmjopen-2015-007961. BMJ Open. 2015. PMID: 26423849 Free PMC article. Review.
-
Anatomy of the epidemiological literature on the 2003 SARS outbreaks in Hong Kong and Toronto: a time-stratified review.PLoS Med. 2010 May 4;7(5):e1000272. doi: 10.1371/journal.pmed.1000272. PLoS Med. 2010. PMID: 20454570 Free PMC article. Review.
Cited by
-
Publication status of completed registered studies in paediatric appendicitis: a cross-sectional analysis.BMJ Open. 2018 Jul 16;8(7):e021684. doi: 10.1136/bmjopen-2018-021684. BMJ Open. 2018. PMID: 30012791 Free PMC article.
-
Clinical trial registration and reporting: a survey of academic organizations in the United States.BMC Med. 2018 May 2;16(1):60. doi: 10.1186/s12916-018-1042-6. BMC Med. 2018. PMID: 29716585 Free PMC article.
-
Publication statuses of clinical trials supporting FDA-approved immune checkpoint inhibitors: a meta-epidemiological investigation.BMC Cancer. 2019 Oct 24;19(1):998. doi: 10.1186/s12885-019-6232-x. BMC Cancer. 2019. PMID: 31651263 Free PMC article.
-
The ENCePP Code of Conduct: A best practise for scientific independence and transparency in noninterventional postauthorisation studies.Pharmacoepidemiol Drug Saf. 2019 Apr;28(4):422-433. doi: 10.1002/pds.4763. Epub 2019 Mar 5. Pharmacoepidemiol Drug Saf. 2019. PMID: 30838708 Free PMC article. Review.
-
Patient consent to publication and data sharing in industry and NIH-funded clinical trials.Trials. 2018 May 3;19(1):269. doi: 10.1186/s13063-018-2651-2. Trials. 2018. PMID: 29724236 Free PMC article.
References
-
- Goldacre B. Bad Pharma: How Drug Companies Mislead Doctors and Harm Patients.Faber and Faber, Inc, 2013.
-
- Mansi BA, Clark J, David FS, et al. Ten recommendations for closing the credibility gap in reporting industry-sponsored clinical research: a joint journal and pharmaceutical industry perspective. Mayo Clin Proc 2012;87:424-9. 10.1016/j.mayocp.2012.02.009 pmid:22560521. - DOI - PMC - PubMed
-
- Miller JE, Korn D, Ross JS. Clinical trial registration, reporting, publication and FDAAA compliance: a cross-sectional analysis and ranking of new drugs approved by the FDA in 2012. BMJ Open 2015;5:e009758 10.1136/bmjopen-2015-009758. pmid:26563214. - DOI - PMC - PubMed
-
- Chen R, Desai NR, Ross JS, et al. Publication and reporting of clinical trial results: cross sectional analysis across academic medical centers. BMJ 2016;352:i637 10.1136/bmj.i637. pmid:26888209. - DOI - PMC - PubMed
-
- Bourgeois FT, Murthy S, Mandl KD. Outcome reporting among drug trials registered in ClinicalTrials.gov. Ann Intern Med 2010;153:158-66. 10.7326/0003-4819-153-3-201008030-00006 pmid:20679560. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources