Genome-Wide Investigation of Biofilm Formation in Bacillus cereus
- PMID: 28432092
- PMCID: PMC5478996
- DOI: 10.1128/AEM.00561-17
Genome-Wide Investigation of Biofilm Formation in Bacillus cereus
Abstract
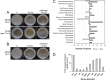
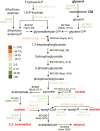
Bacillus cereus is a soil-dwelling Gram-positive bacterium capable of forming structured multicellular communities, or biofilms. However, the regulatory pathways controlling biofilm formation are less well understood in B. cereus In this work, we developed a method to study B. cereus biofilms formed at the air-liquid interface. We applied two genome-wide approaches, random transposon insertion mutagenesis to identify genes that are potentially important for biofilm formation, and transcriptome analyses by RNA sequencing (RNA-seq) to characterize genes that are differentially expressed in B. cereus when cells were grown in a biofilm-inducing medium. For the first approach, we identified 23 genes whose disruption by transposon insertion led to altered biofilm phenotypes. Based on the predicted function, they included genes involved in processes such as nucleotide biosynthesis, iron salvage, and antibiotic production, as well as genes encoding an ATP-dependent protease and transcription regulators. Transcriptome analyses identified about 500 genes that were differentially expressed in cells grown under biofilm-inducing conditions. One particular set of those genes may contribute to major metabolic shifts, leading to elevated production of small volatile molecules. Selected volatile molecules were shown to stimulate robust biofilm formation in B. cereus Our studies represent a genome-wide investigation of B. cereus biofilm formation.IMPORTANCE In this work, we established a robust method for B. cereus biofilm studies and applied two genome-wide approaches, transposon insertion mutagenesis and transcriptome analyses by RNA-seq, to identify genes and pathways that are potentially important for biofilm formation in B. cereus We discovered dozens of genes and two major metabolic shifts that seem to be important for biofilm formation in B. cereus Our study represents a genome-wide investigation on B. cereus biofilm formation.
Keywords: Bacillus cereus; biofilm formation; transcriptome; transposon mutagenesis.
Copyright © 2017 American Society for Microbiology.
Figures




References
-
- Chen Y, Yan F, Chai Y, Liu H, Kolter R, Losick R, Guo J-h. 2013. Biocontrol of tomato wilt disease by Bacillus subtilis isolates from natural environments depends on conserved genes mediating biofilm formation. Environ Microbiol 15:848–864. doi: 10.1111/j.1462-2920.2012.02860.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

