Two Distinct α-l-Arabinofuranosidases in Caldicellulosiruptor Species Drive Degradation of Arabinose-Based Polysaccharides
- PMID: 28432102
- PMCID: PMC5479001
- DOI: 10.1128/AEM.00574-17
Two Distinct α-l-Arabinofuranosidases in Caldicellulosiruptor Species Drive Degradation of Arabinose-Based Polysaccharides
Abstract
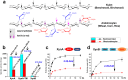
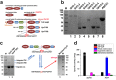
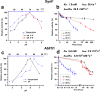
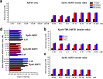
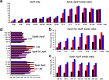
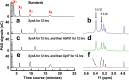
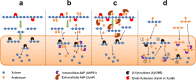
Species in the extremely thermophilic genus Caldicellulosiruptor can degrade unpretreated plant biomass through the action of multimodular glycoside hydrolases. To date, most focus with these bacteria has been on hydrolysis of glucans and xylans, while the biodegradation mechanism for arabinose-based polysaccharides remains unclear. Here, putative α-l-arabinofuranosidases (AbFs) were identified in Caldicellulosiruptor species by homology to less-thermophilic versions of these enzymes. From this screen, an extracellular XynF was determined to be a key factor in hydrolyzing α-1,2-, α-1,3-, and α-1,5-l-arabinofuranosyl residues of arabinose-based polysaccharides. Combined with a GH11 xylanase (XynA), XynF increased arabinoxylan hydrolysis more than 6-fold compared to the level seen with XynA alone, likely the result of XynF removing arabinofuranosyl side chains to generate linear xylans that were readily degraded. A second AbF, the intracellular AbF51, preferentially cleaved the α-1,5-l-arabinofuranosyl glycoside bonds within sugar beet arabinan. β-Xylosidases, such as GH39 Xyl39B, facilitated the hydrolysis of arabinofuranosyl residues at the nonreducing terminus of the arabinose-branched xylo-oligosaccharides by AbF51. These results demonstrate the separate but complementary contributions of extracellular XynF and cytosolic AbF51 in processing the bioconversion of arabinose-containing oligosaccharides to fermentable monosaccharides.IMPORTANCE Degradation of hemicellulose, due to its complex chemical structure, presents a major challenge during bioconversion of lignocellulosic biomass to biobased fuels and chemicals. Degradation of arabinose-containing polysaccharides, in particular, can be a key bottleneck in this process. Among Caldicellulosiruptor species, the multimodular arabinofuranosidase XynF is present in only selected members of this genus. This enzyme exhibited high hydrolysis activity, broad specificity, and strong synergism with other hemicellulases acting on arabino-polysaccharides. An intracellular arabinofuranosidase, AbF51, occurs in all Caldicellulosiruptor species and, in conjunction with xylosidases, processes the bioconversion of arabinose-branched oligosaccharides to fermentable monosaccharides. Taken together, the data suggest that plant biomass degradation in Caldicellulosiruptor species involves extracellular XynF that acts synergistically with other hemicellulases to digest arabino-polysaccharides that are subsequently transported and degraded further by intracellular AbF51 to produce short-chain arabino sugars.
Keywords: Arabinofuranosidase; bioenergy; glycoside hydrolase; hyperthermophiles; synergism.
Copyright © 2017 American Society for Microbiology.
Figures



Similar articles
-
Two Novel α-l-Arabinofuranosidases from Bifidobacterium longum subsp. longum Belonging to Glycoside Hydrolase Family 43 Cooperatively Degrade Arabinan.Appl Environ Microbiol. 2019 Mar 6;85(6):e02582-18. doi: 10.1128/AEM.02582-18. Print 2019 Mar 15. Appl Environ Microbiol. 2019. PMID: 30635377 Free PMC article.
-
Functional Analysis of the Glucan Degradation Locus in Caldicellulosiruptor bescii Reveals Essential Roles of Component Glycoside Hydrolases in Plant Biomass Deconstruction.Appl Environ Microbiol. 2017 Dec 1;83(24):e01828-17. doi: 10.1128/AEM.01828-17. Print 2017 Dec 15. Appl Environ Microbiol. 2017. PMID: 28986379 Free PMC article.
-
Identification and characterization of GH62 bacterial α-l-arabinofuranosidase from thermotolerant Streptomyces sp. SWU10 that preferentially degrades branched l-arabinofuranoses in wheat arabinoxylan.Enzyme Microb Technol. 2018 May;112:22-28. doi: 10.1016/j.enzmictec.2018.01.009. Epub 2018 Jan 31. Enzyme Microb Technol. 2018. PMID: 29499776
-
Microbial α-L-arabinofuranosidases: diversity, properties, and biotechnological applications.World J Microbiol Biotechnol. 2024 Jan 31;40(3):84. doi: 10.1007/s11274-023-03882-z. World J Microbiol Biotechnol. 2024. PMID: 38294733 Review.
-
GH62 arabinofuranosidases: Structure, function and applications.Biotechnol Adv. 2017 Nov 1;35(6):792-804. doi: 10.1016/j.biotechadv.2017.06.005. Epub 2017 Jun 29. Biotechnol Adv. 2017. PMID: 28669588 Review.
Cited by
-
Biochemical and Molecular Dynamics Study of a Novel GH 43 α-l-Arabinofuranosidase/β-Xylosidase From Caldicellulosiruptor saccharolyticus DSM8903.Front Bioeng Biotechnol. 2022 Feb 11;10:810542. doi: 10.3389/fbioe.2022.810542. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35223784 Free PMC article.
-
Discovery of a bifunctional xylanolytic enzyme with arabinoxylan arabinofuranohydrolase-d3 and endo-xylanase activities and its application in the hydrolysis of cereal arabinoxylans.Microb Biotechnol. 2023 Jul;16(7):1536-1547. doi: 10.1111/1751-7915.14267. Epub 2023 Apr 25. Microb Biotechnol. 2023. PMID: 37096984 Free PMC article.
-
Arabinan hydrolysis by GH43 enzymes of Hungateiclostridium clariflavum and the potential synergistic mechanisms.Appl Microbiol Biotechnol. 2022 Dec;106(23):7793-7803. doi: 10.1007/s00253-022-12238-w. Epub 2022 Oct 17. Appl Microbiol Biotechnol. 2022. PMID: 36251023
-
Exploration of Two Pectate Lyases from Caldicellulosiruptor bescii Reveals that the CBM66 Module Has a Crucial Role in Pectic Biomass Degradation.Appl Environ Microbiol. 2020 Aug 3;86(16):e00787-20. doi: 10.1128/AEM.00787-20. Print 2020 Aug 3. Appl Environ Microbiol. 2020. PMID: 32532871 Free PMC article.
-
Distinct roles of carbohydrate-binding modules in multidomain β-1,3-1,4-glucanase on polysaccharide degradation.Appl Microbiol Biotechnol. 2023 Mar;107(5-6):1751-1764. doi: 10.1007/s00253-023-12416-4. Epub 2023 Feb 17. Appl Microbiol Biotechnol. 2023. PMID: 36800030
References
-
- Shallom D, Belakhov V, Solomon D, Gilead-Gropper S, Baasov T, Shoham G, Shoham Y. 2002. The identification of the acid-base catalyst of α-arabinofuranosidase from Geobacillus stearothermophilus T-6, a family 51 glycoside hydrolase. FEBS Lett 514:163–167. doi:10.1016/S0014-5793(02)02343-8. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

