Dual regulation of Stat1 and Stat3 by the tumor suppressor protein PML contributes to interferon α-mediated inhibition of angiogenesis
- PMID: 28432122
- PMCID: PMC5473212
- DOI: 10.1074/jbc.M116.771071
Dual regulation of Stat1 and Stat3 by the tumor suppressor protein PML contributes to interferon α-mediated inhibition of angiogenesis
Abstract
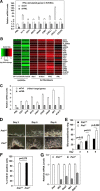
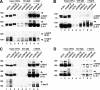
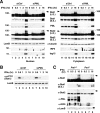
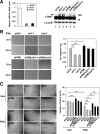
IFNs are effective in inhibiting angiogenesis in preclinical models and in treating several angioproliferative disorders. However, the detailed mechanisms of IFNα-mediated anti-angiogenesis are not completely understood. Stat1/2/3 and PML are IFNα downstream effectors and are pivotal regulators of angiogenesis. Here, we investigated PML's role in the regulation of Stat1/2/3 activity. In Pml knock-out (KO) mice, ablation of Pml largely reduces IFNα angiostatic ability in Matrigel plug assays. This suggested an essential role for PML in IFNα's anti-angiogenic function. We also demonstrated that PML shared a large cohort of regulatory genes with Stat1 and Stat3, indicating an important role of PML in regulating Stat1 and Stat3 activity. Using molecular tools and primary endothelial cells, we demonstrated that PML positively regulates Stat1 and Stat2 isgylation, a ubiquitination-like protein modification. Accordingly, manipulation of the isgylation system by knocking down USP18 altered IFNα-PML axis-mediated inhibition of endothelial cell migration and network formation. Furthermore, PML promotes turnover of nuclear Stat3, and knockdown of PML mitigates the effect of LLL12, a selective Stat3 inhibitor, on IFNα-mediated anti-angiogenic activity. Taken together, we elucidated an unappreciated mechanism in which PML, an IFNα-inducible effector, possess potent angiostatic activity, doing so in part by forming a positive feedforward loop with Stat1/2 and a negative feedback loop with Stat3. The interplay between PML, Stat1/Stat2, and Stat3 contributes to IFNα-mediated inhibition of angiogenesis, and disruption of this network results in aberrant IFNα signaling and altered angiostatic activity.
Keywords: STAT transcription factor; STAT3; angiogenesis; interferon; signal transducers and activators of transcription 1 (STAT1).
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




Similar articles
-
Promyelocytic leukemia protein (PML) regulates endothelial cell network formation and migration in response to tumor necrosis factor α (TNFα) and interferon α (IFNα).J Biol Chem. 2012 Jul 6;287(28):23356-67. doi: 10.1074/jbc.M112.340505. Epub 2012 May 15. J Biol Chem. 2012. PMID: 22589541 Free PMC article.
-
Disentangling molecular mechanisms regulating sensitization of interferon alpha signal transduction.Mol Syst Biol. 2020 Jul;16(7):e8955. doi: 10.15252/msb.20198955. Mol Syst Biol. 2020. PMID: 32696599 Free PMC article.
-
Role of STAT3 in type I interferon responses. Negative regulation of STAT1-dependent inflammatory gene activation.J Biol Chem. 2006 May 19;281(20):14111-8. doi: 10.1074/jbc.M511797200. Epub 2006 Mar 29. J Biol Chem. 2006. PMID: 16571725
-
STAT proteins as novel targets for cancer drug discovery.Expert Opin Ther Targets. 2004 Oct;8(5):409-22. doi: 10.1517/14728222.8.5.409. Expert Opin Ther Targets. 2004. PMID: 15469392 Review.
-
The functions of signal transducers and activators of transcriptions 1 and 3 as cytokine-inducible proteins.J Interferon Cytokine Res. 2011 Jan;31(1):33-40. doi: 10.1089/jir.2010.0100. Epub 2010 Dec 19. J Interferon Cytokine Res. 2011. PMID: 21166594 Free PMC article. Review.
Cited by
-
The HECT E3 Ligase E6AP/UBE3A as a Therapeutic Target in Cancer and Neurological Disorders.Cancers (Basel). 2020 Jul 29;12(8):2108. doi: 10.3390/cancers12082108. Cancers (Basel). 2020. PMID: 32751183 Free PMC article. Review.
-
The complementary roles of STAT3 and STAT1 in cancer biology: insights into tumor pathogenesis and therapeutic strategies.Front Immunol. 2023 Nov 8;14:1265818. doi: 10.3389/fimmu.2023.1265818. eCollection 2023. Front Immunol. 2023. PMID: 38022653 Free PMC article. Review.
-
Revealing nuclear receptor hub modules from Basal-like breast cancer expression networks.PLoS One. 2021 Jun 23;16(6):e0252901. doi: 10.1371/journal.pone.0252901. eCollection 2021. PLoS One. 2021. PMID: 34161324 Free PMC article.
-
TRIMming Down Hormone-Driven Cancers: The Biological Impact of TRIM Proteins on Tumor Development, Progression and Prognostication.Cells. 2021 Jun 16;10(6):1517. doi: 10.3390/cells10061517. Cells. 2021. PMID: 34208621 Free PMC article. Review.
-
Adverse Cardiovascular Effects of Anti-COVID-19 Drugs.Front Pharmacol. 2021 Aug 25;12:699949. doi: 10.3389/fphar.2021.699949. eCollection 2021. Front Pharmacol. 2021. PMID: 34512335 Free PMC article. Review.
References
-
- Liu F., Gao X., Wang J., Gao C., Li X., Li X., Gong X., and Zeng X. (2016) Transcriptome sequencing to identify transcription factor regulatory network and alternative splicing in endothelial cells under VEGF stimulation. J. Mol. Neurosci. 58, 170–177 - PubMed
-
- Sgonc R., Fuerhapter C., Boeck G., Swerlick R., Fritsch P., and Sepp N. (1998) Induction of apoptosis in human dermal microvascular endothelial cells and infantile hemangiomas by interferon-α. Int. Arch. Allergy Immunol. 117, 209–214 - PubMed
-
- Albini A., Marchisone C., Del Grosso F., Benelli R., Masiello L., Tacchetti C., Bono M., Ferrantini M., Rozera C., Truini M., Belardelli F., Santi L., and Noonan D. M. (2000) Inhibition of angiogenesis and vascular tumor growth by interferon-producing cells: a gene therapy approach. Am. J. Pathol. 156, 1381–1393 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

