An amphipathic α-helix directs palmitoylation of the large intracellular loop of the sodium/calcium exchanger
- PMID: 28432123
- PMCID: PMC5481580
- DOI: 10.1074/jbc.M116.773945
An amphipathic α-helix directs palmitoylation of the large intracellular loop of the sodium/calcium exchanger
Abstract
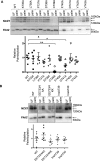
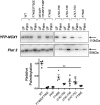
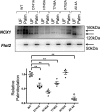
The electrogenic sodium/calcium exchanger (NCX) mediates bidirectional calcium transport controlled by the transmembrane sodium gradient. NCX inactivation occurs in the absence of phosphatidylinositol 4,5-bisphosphate and is facilitated by palmitoylation of a single cysteine at position 739 within the large intracellular loop of NCX. The aim of this investigation was to identify the structural determinants of NCX1 palmitoylation. Full-length NCX1 (FL-NCX1) and a YFP fusion protein of the NCX1 large intracellular loop (YFP-NCX1) were expressed in HEK cells. Single amino acid changes around Cys-739 in FL-NCX1 and deletions on the N-terminal side of Cys-739 in YFP-NCX1 did not affect NCX1 palmitoylation, with the exception of the rare human polymorphism S738F, which enhanced FL-NCX1 palmitoylation, and D741A, which modestly reduced it. In contrast, deletion of a 21-amino acid segment enriched in aromatic amino acids on the C-terminal side of Cys-739 abolished YFP-NCX1 palmitoylation. We hypothesized that this segment forms an amphipathic α-helix whose properties facilitate Cys-739 palmitoylation. Introduction of negatively charged amino acids to the hydrophobic face or of helix-breaking prolines impaired palmitoylation of both YFP-NCX1 and FL-NCX1. Alanine mutations on the hydrophilic face of the helix significantly reduced FL-NCX1 palmitoylation. Of note, when the helix-containing segment was introduced adjacent to cysteines that are not normally palmitoylated, they became palmitoylation sites. In conclusion, we have identified an amphipathic α-helix in the NCX1 large intracellular loop that controls NCX1 palmitoylation. NCX1 palmitoylation is governed by a distal secondary structure element rather than by local primary sequence.
Keywords: acyltransferase; calcium transport; protein acylation; protein palmitoylation; sodium transport; sodium-calcium exchange.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



Similar articles
-
Dynamic Palmitoylation of the Sodium-Calcium Exchanger Modulates Its Structure, Affinity for Lipid-Ordered Domains, and Inhibition by XIP.Cell Rep. 2020 Jun 9;31(10):107697. doi: 10.1016/j.celrep.2020.107697. Cell Rep. 2020. PMID: 32521252 Free PMC article.
-
Palmitoylation of the Na/Ca exchanger cytoplasmic loop controls its inactivation and internalization during stress signaling.FASEB J. 2015 Nov;29(11):4532-43. doi: 10.1096/fj.15-276493. Epub 2015 Jul 14. FASEB J. 2015. PMID: 26174834 Free PMC article.
-
Regulation of NCX1 by palmitoylation.Cell Calcium. 2020 Mar;86:102158. doi: 10.1016/j.ceca.2019.102158. Epub 2020 Jan 8. Cell Calcium. 2020. PMID: 31935590 Review.
-
Topical review: Shedding light on molecular and cellular consequences of NCX1 palmitoylation.Cell Signal. 2020 Dec;76:109791. doi: 10.1016/j.cellsig.2020.109791. Epub 2020 Sep 24. Cell Signal. 2020. PMID: 32980495 Review.
-
Rise of palmitoylation: A new trick to tune NCX1 activity.Biochim Biophys Acta Mol Cell Res. 2024 Jun;1871(5):119719. doi: 10.1016/j.bbamcr.2024.119719. Epub 2024 Apr 3. Biochim Biophys Acta Mol Cell Res. 2024. PMID: 38574822 Review.
Cited by
-
The role of an amphiphilic helix and transmembrane region in the efficient acylation of the M2 protein from influenza virus.Sci Rep. 2023 Nov 2;13(1):18928. doi: 10.1038/s41598-023-45945-z. Sci Rep. 2023. PMID: 37919373 Free PMC article.
-
Palmitoylation of solute carriers.Biochem Pharmacol. 2023 Sep;215:115695. doi: 10.1016/j.bcp.2023.115695. Epub 2023 Jul 20. Biochem Pharmacol. 2023. PMID: 37481134 Free PMC article. Review.
-
Dynamic Palmitoylation of the Sodium-Calcium Exchanger Modulates Its Structure, Affinity for Lipid-Ordered Domains, and Inhibition by XIP.Cell Rep. 2020 Jun 9;31(10):107697. doi: 10.1016/j.celrep.2020.107697. Cell Rep. 2020. PMID: 32521252 Free PMC article.
-
Proton-modulated interactions of ions with transport sites of prokaryotic and eukaryotic NCX prototypes.Cell Calcium. 2021 Nov;99:102476. doi: 10.1016/j.ceca.2021.102476. Epub 2021 Sep 20. Cell Calcium. 2021. PMID: 34564055 Free PMC article.
-
S-Palmitoylation of the sodium channel Nav1.6 regulates its activity and neuronal excitability.J Biol Chem. 2020 May 1;295(18):6151-6164. doi: 10.1074/jbc.RA119.012423. Epub 2020 Mar 11. J Biol Chem. 2020. PMID: 32161114 Free PMC article.
References
-
- Philipson K. D., and Nicoll D. A. (2000) Sodium-calcium exchange: a molecular perspective. Annu. Rev. Physiol. 62, 111–133 - PubMed
-
- Lytton J. (2007) Na+/Ca2+ exchangers: three mammalian gene families control Ca2+ transport. Biochem. J. 406, 365–382 - PubMed
-
- Liao J., Li H., Zeng W., Sauer D. B., Belmares R., and Jiang Y. (2012) Structural insight into the ion-exchange mechanism of the sodium/calcium exchanger. Science 335, 686–690 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

