TGF-β1 Signaling and Tissue Fibrosis
- PMID: 28432134
- PMCID: PMC5880172
- DOI: 10.1101/cshperspect.a022293
TGF-β1 Signaling and Tissue Fibrosis
Abstract
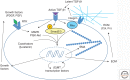
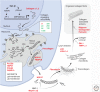
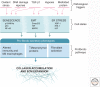
Activation of TGF-β1 initiates a program of temporary collagen accumulation important to wound repair in many organs. However, the outcome of temporary extracellular matrix strengthening all too frequently morphs into progressive fibrosis, contributing to morbidity and mortality worldwide. To avoid this maladaptive outcome, TGF-β1 signaling is regulated at numerous levels and intimately connected to feedback signals that limit accumulation. Here, we examine the current understanding of the core functions of TGF-β1 in promoting collagen accumulation, parallel pathways that promote physiological repair, and pathological triggers that tip the balance toward progressive fibrosis. Implicit in better understanding of these processes is the identification of therapeutic opportunities that will need to be further advanced to limit or reverse organ fibrosis.
Copyright © 2018 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
References
-
- Al-Alawi M, Hassan T, Chotirmall SH. 2014. Transforming growth factor β and severe asthma: A perfect storm. Respir Med 108: 1409–1423. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
