Focal Local Field Potential Signature of the Single-Axon Monosynaptic Thalamocortical Connection
- PMID: 28432143
- PMCID: PMC5444196
- DOI: 10.1523/JNEUROSCI.2715-16.2017
Focal Local Field Potential Signature of the Single-Axon Monosynaptic Thalamocortical Connection
Abstract
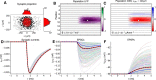
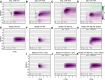
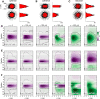
A resurgence has taken place in recent years in the use of the extracellularly recorded local field potential (LFP) to investigate neural network activity. To probe monosynaptic thalamic activation of cortical postsynaptic target cells, so called spike-trigger-averaged LFP (stLFP) signatures have been measured. In these experiments, the cortical LFP is measured by multielectrodes covering several cortical lamina and averaged on spontaneous spikes of thalamocortical (TC) cells. Using a well established forward-modeling scheme, we investigated the biophysical origin of this stLFP signature with simultaneous synaptic activation of cortical layer-4 neurons, mimicking the effect of a single afferent spike from a single TC neuron. Constrained by previously measured intracellular responses of the main postsynaptic target cell types and with biologically plausible assumptions regarding the spatial distribution of thalamic synaptic inputs into layer 4, the model predicted characteristic contributions to monosynaptic stLFP signatures both for the regular-spiking (RS) excitatory neurons and the fast-spiking (FS) inhibitory interneurons. In particular, the FS cells generated stLFP signatures of shorter temporal duration than the RS cells. Added together, a sum of the stLFP signatures of these two principal synaptic targets of TC cells were observed to resemble experimentally measured stLFP signatures. Outside the volume targeted by TC afferents, the resulting postsynaptic LFP signals were found to be sharply attenuated. This implies that such stLFP signatures provide a very local measure of TC synaptic activation, and that newly developed inverse current-source density (CSD)-estimation methods are needed for precise assessment of the underlying spatiotemporal CSD profiles.SIGNIFICANCE STATEMENT Despite its long history and prevalent use, the proper interpretation of the extracellularly recorded local field potential (LFP) is still not fully established. Here we investigate by biophysical modeling the origin of the focal LFP signature of the single-axon monosynaptic thalamocortical connection as measured by spike-trigger-averaging of cortical LFPs on spontaneous spikes of thalamocortical neurons. We find that this LFP signature is well accounted for by a model assuming thalamic projections to two cortical layer-4 cell populations: one excitatory (putatively regular-spiking cells) and one inhibitory (putatively fast-spiking cells). The LFP signature is observed to decay sharply outside the cortical region receiving the thalamocortical projection, implying that it indeed provides a very local measure of thalamocortical synaptic activation.
Keywords: layer 4; local field potential; modeling; monosynaptic; sensory cortex; thalamocortical.
Copyright © 2017 Hagen et al.
Figures







Similar articles
-
Spike timing and synaptic dynamics at the awake thalamocortical synapse.Prog Brain Res. 2005;149:91-105. doi: 10.1016/S0079-6123(05)49008-1. Prog Brain Res. 2005. PMID: 16226579 Review.
-
Differential Excitation of Distally versus Proximally Targeting Cortical Interneurons by Unitary Thalamocortical Bursts.J Neurosci. 2016 Jun 29;36(26):6906-16. doi: 10.1523/JNEUROSCI.0739-16.2016. J Neurosci. 2016. PMID: 27358449 Free PMC article.
-
Short-term dynamics of thalamocortical and intracortical synapses onto layer 6 neurons in neocortex.J Neurophysiol. 2002 Oct;88(4):1924-32. doi: 10.1152/jn.2002.88.4.1924. J Neurophysiol. 2002. PMID: 12364518
-
Feedforward inhibitory connections from multiple thalamic cells to multiple regular-spiking cells in layer 4 of the somatosensory cortex.J Neurophysiol. 2006 Oct;96(4):1746-54. doi: 10.1152/jn.00301.2006. Epub 2006 Jul 19. J Neurophysiol. 2006. PMID: 16855112
-
Thalamocortical control of feed-forward inhibition in awake somatosensory 'barrel' cortex.Philos Trans R Soc Lond B Biol Sci. 2002 Dec 29;357(1428):1717-27. doi: 10.1098/rstb.2002.1156. Philos Trans R Soc Lond B Biol Sci. 2002. PMID: 12626006 Free PMC article. Review.
Cited by
-
Brain signal predictions from multi-scale networks using a linearized framework.PLoS Comput Biol. 2022 Aug 12;18(8):e1010353. doi: 10.1371/journal.pcbi.1010353. eCollection 2022 Aug. PLoS Comput Biol. 2022. PMID: 35960767 Free PMC article.
-
Multielectrodes join the connectome.Nat Methods. 2017 Sep;14(9):847-848. doi: 10.1038/nmeth.4424. Nat Methods. 2017. PMID: 28858341 No abstract available.
-
Cortical origin of theta error signals.Cereb Cortex. 2023 Nov 27;33(23):11300-11319. doi: 10.1093/cercor/bhad367. Cereb Cortex. 2023. PMID: 37804250 Free PMC article.
-
Large-scale mapping of cortical synaptic projections with extracellular electrode arrays.Nat Methods. 2017 Sep;14(9):882-890. doi: 10.1038/nmeth.4393. Epub 2017 Aug 14. Nat Methods. 2017. PMID: 28805794
-
On the validity of electric brain signal predictions based on population firing rates.PLoS Comput Biol. 2025 Apr 14;21(4):e1012303. doi: 10.1371/journal.pcbi.1012303. eCollection 2025 Apr. PLoS Comput Biol. 2025. PMID: 40228210 Free PMC article.
References
-
- Ascoli GA, Donohue DE, Halavi M (2007) NeuroMorpho.Org: a central resource for neuronal morphologies. J Neurosci 27:9247–9251. 10.1523/JNEUROSCI.2055-07.2007 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
