Gene expression effects of glucocorticoid and mineralocorticoid receptor agonists and antagonists on normal human skeletal muscle
- PMID: 28432191
- PMCID: PMC5495910
- DOI: 10.1152/physiolgenomics.00128.2016
Gene expression effects of glucocorticoid and mineralocorticoid receptor agonists and antagonists on normal human skeletal muscle
Abstract
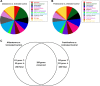
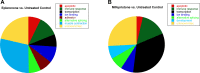
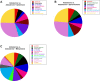
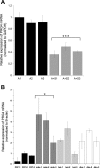
Mineralocorticoid and glucocorticoid receptors are closely related steroid hormone receptors that regulate gene expression through many of the same hormone response elements. However, their transcriptional activities and effects in skeletal muscles are largely unknown. We recently identified mineralocorticoid receptors (MR) in skeletal muscles after finding that combined treatment with the angiotensin-converting enzyme inhibitor lisinopril and MR antagonist spironolactone was therapeutic in Duchenne muscular dystrophy mouse models. The glucocorticoid receptor (GR) agonist prednisolone is the current standard-of-care treatment for Duchenne muscular dystrophy because it prolongs ambulation, likely due to its anti-inflammatory effects. However, data on whether glucocorticoids have a beneficial or detrimental direct effect on skeletal muscle are controversial. Here, we begin to define the gene expression profiles in normal differentiated human skeletal muscle myotubes treated with MR and GR agonists and antagonists. The MR agonist aldosterone and GR agonist prednisolone had highly overlapping gene expression profiles, supporting the notion that prednisolone acts as both a GR and MR agonist that may have detrimental effects on skeletal muscles. Co-incubations with aldosterone plus either nonspecific or selective MR antagonists, spironolactone or eplerenone, resulted in similar numbers of gene expression changes, suggesting that both drugs can block MR activation to a similar extent. Eplerenone treatment alone decreased a number of important muscle-specific genes. This information may be used to develop biomarkers to monitor clinical efficacy of MR antagonists or GR agonists in muscular dystrophy, develop a temporally coordinated treatment with both drugs, or identify novel therapeutics with more specific downstream targets.
Keywords: Duchenne muscular dystrophy; aldosterone; eplerenone; glucocorticoid receptor; mifepristone; mineralocorticoid receptor; prednisolone; spironolactone.
Copyright © 2017 the American Physiological Society.
Figures

Similar articles
-
Similar efficacy from specific and non-specific mineralocorticoid receptor antagonist treatment of muscular dystrophy mice.J Neuromuscul Dis. 2016;3(3):395-404. doi: 10.3233/JND-160173. J Neuromuscul Dis. 2016. PMID: 27822449 Free PMC article.
-
Renin-angiotensin-aldosterone system inhibitors improve membrane stability and change gene-expression profiles in dystrophic skeletal muscles.Am J Physiol Cell Physiol. 2017 Feb 1;312(2):C155-C168. doi: 10.1152/ajpcell.00269.2016. Epub 2016 Nov 23. Am J Physiol Cell Physiol. 2017. PMID: 27881412 Free PMC article.
-
Mineralocorticoid receptors are present in skeletal muscle and represent a potential therapeutic target.FASEB J. 2015 Nov;29(11):4544-54. doi: 10.1096/fj.15-276782. Epub 2015 Jul 15. FASEB J. 2015. PMID: 26178166 Free PMC article.
-
Towards selectively modulating mineralocorticoid receptor function: lessons from other systems.Mol Cell Endocrinol. 2004 Mar 31;217(1-2):151-65. doi: 10.1016/j.mce.2003.10.044. Mol Cell Endocrinol. 2004. PMID: 15134814 Review.
-
Mineralocorticoid Receptor Signaling in the Inflammatory Skeletal Muscle Microenvironments of Muscular Dystrophy and Acute Injury.Front Pharmacol. 2022 Jun 28;13:942660. doi: 10.3389/fphar.2022.942660. eCollection 2022. Front Pharmacol. 2022. PMID: 35837290 Free PMC article. Review.
Cited by
-
Phase 1 trial of vamorolone, a first-in-class steroid, shows improvements in side effects via biomarkers bridged to clinical outcomes.Steroids. 2018 Jun;134:43-52. doi: 10.1016/j.steroids.2018.02.010. Epub 2018 Mar 8. Steroids. 2018. PMID: 29524454 Free PMC article. Clinical Trial.
-
Mineralocorticoid receptor antagonists improve membrane integrity independent of muscle force in muscular dystrophy.Hum Mol Genet. 2019 Jun 15;28(12):2030-2045. doi: 10.1093/hmg/ddz039. Hum Mol Genet. 2019. PMID: 30759207 Free PMC article.
-
Synergistic Effects of Multiple Factors Involved in COVID-19-dependent Muscle Loss.Aging Dis. 2022 Apr 1;13(2):344-352. doi: 10.14336/AD.2021.0817. eCollection 2022 Apr. Aging Dis. 2022. PMID: 35371610 Free PMC article.
-
Myeloid mineralocorticoid receptors contribute to skeletal muscle repair in muscular dystrophy and acute muscle injury.Am J Physiol Cell Physiol. 2022 Mar 1;322(3):C354-C369. doi: 10.1152/ajpcell.00411.2021. Epub 2022 Jan 19. Am J Physiol Cell Physiol. 2022. PMID: 35044859 Free PMC article.
-
Mineralocorticoid Receptor Signaling Contributes to Normal Muscle Repair After Acute Injury.Front Physiol. 2019 Oct 25;10:1324. doi: 10.3389/fphys.2019.01324. eCollection 2019. Front Physiol. 2019. PMID: 31736768 Free PMC article.
References
-
- Amazit L, Le Billan F, Kolkhof P, Lamribet K, Viengchareun S, Fay MR, Khan JA, Hillisch A, Lombès M, Rafestin-Oblin ME, Fagart J. Finerenone impedes aldosterone-dependent nuclear import of the mineralocorticoid receptor and prevents genomic recruitment of steroid receptor coactivator-1. J Biol Chem 290: 21876–21889, 2015. doi:10.1074/jbc.M115.657957. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

