Folate-dependent methylation of septins governs ciliogenesis during neural tube closure
- PMID: 28432198
- PMCID: PMC5503710
- DOI: 10.1096/fj.201700092R
Folate-dependent methylation of septins governs ciliogenesis during neural tube closure
Abstract
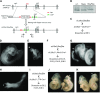
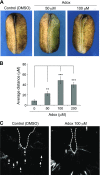
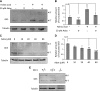
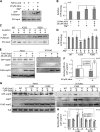
Periconception maternal folic acid (vitamin B9) supplementation can reduce the prevalence of neural tube defects (NTDs), although just how folates benefit the developing embryo and promote closing of the neural tube and other morphologic processes during development remains unknown. Folate contributes to a 1-carbon metabolism, which is essential for purine biosynthesis and methionine recycling and affects methylation of DNA, histones, and nonhistone proteins. Herein, we used animal models and cultured mammalian cells to demonstrate that disruption of the methylation pathway mediated by folate compromises normal neural tube closure (NTC) and ciliogenesis. We demonstrate that the embryos with NTD failed to adequately methylate septin2, a key regulator of cilium structure and function. We report that methylation of septin2 affected its GTP binding activity and formation of the septin2-6-7 complex. We propose that folic acid promotes normal NTC in some embryos by regulating the methylation of septin2, which is critical for normal cilium formation during early embryonic development.-Toriyama, M., Toriyama, M., Wallingford, J. B., Finnell, R. H. Folate-dependent methylation of septins governs ciliogenesis during neural tube closure.
Keywords: epigenetics; folate transport; neural tube defect; septin2.
© FASEB.
Figures




Similar articles
-
Folate-mediated one-carbon metabolism and neural tube defects: balancing genome synthesis and gene expression.Birth Defects Res C Embryo Today. 2007 Sep;81(3):183-203. doi: 10.1002/bdrc.20100. Birth Defects Res C Embryo Today. 2007. PMID: 17963270 Review.
-
Folate receptors and neural tube closure.Congenit Anom (Kyoto). 2017 Sep;57(5):130-133. doi: 10.1111/cga.12218. Epub 2017 Apr 18. Congenit Anom (Kyoto). 2017. PMID: 28244241 Review.
-
Maternal dietary uridine causes, and deoxyuridine prevents, neural tube closure defects in a mouse model of folate-responsive neural tube defects.Am J Clin Nutr. 2015 Apr;101(4):860-9. doi: 10.3945/ajcn.114.097279. Epub 2015 Jan 28. Am J Clin Nutr. 2015. PMID: 25833982 Free PMC article.
-
Folate deficiency induced H2A ubiquitination to lead to downregulated expression of genes involved in neural tube defects.Epigenetics Chromatin. 2019 Nov 13;12(1):69. doi: 10.1186/s13072-019-0312-7. Epigenetics Chromatin. 2019. PMID: 31722724 Free PMC article.
-
Nutri-epigenomic Studies Related to Neural Tube Defects: Does Folate Affect Neural Tube Closure Via Changes in DNA Methylation?Mini Rev Med Chem. 2015;15(13):1095-102. doi: 10.2174/1389557515666150909144828. Mini Rev Med Chem. 2015. PMID: 26349489 Review.
Cited by
-
SCYL1 arginine methylation by PRMT1 is essential for neurite outgrowth via Golgi morphogenesis.Mol Biol Cell. 2020 Aug 15;31(18):1963-1973. doi: 10.1091/mbc.E20-02-0100. Epub 2020 Jun 17. Mol Biol Cell. 2020. PMID: 32583741 Free PMC article.
-
Spina Bifida: A Review of the Genetics, Pathophysiology and Emerging Cellular Therapies.J Dev Biol. 2022 Jun 6;10(2):22. doi: 10.3390/jdb10020022. J Dev Biol. 2022. PMID: 35735913 Free PMC article. Review.
-
Decreased global DNA hydroxymethylation in neural tube defects: Association with polycyclic aromatic hydrocarbons.Epigenetics. 2019 Oct;14(10):1019-1029. doi: 10.1080/15592294.2019.1629233. Epub 2019 Jun 14. Epigenetics. 2019. PMID: 31179819 Free PMC article.
-
Insights into the Etiology of Mammalian Neural Tube Closure Defects from Developmental, Genetic and Evolutionary Studies.J Dev Biol. 2018 Aug 21;6(3):22. doi: 10.3390/jdb6030022. J Dev Biol. 2018. PMID: 30134561 Free PMC article. Review.
-
Maternal metabolism influences neural tube closure.Trends Endocrinol Metab. 2023 Sep;34(9):539-553. doi: 10.1016/j.tem.2023.06.005. Epub 2023 Jul 17. Trends Endocrinol Metab. 2023. PMID: 37468429 Free PMC article. Review.
References
-
- Golden J. A., Chernoff G. F. (1993) Intermittent pattern of neural tube closure in two strains of mice. Teratology 47, 73–80 - PubMed
-
- Karfunkel P. (1974) The mechanisms of neural tube formation. Int. Rev. Cytol. 38, 245–271 - PubMed
-
- Copp A. J., Greene N. D., Murdoch J. N. (2003) The genetic basis of mammalian neurulation. Nat. Rev. Genet. 4, 784–793 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

