Quiescence of adult oligodendrocyte precursor cells requires thyroid hormone and hypoxia to activate Runx1
- PMID: 28432293
- PMCID: PMC5430791
- DOI: 10.1038/s41598-017-01023-9
Quiescence of adult oligodendrocyte precursor cells requires thyroid hormone and hypoxia to activate Runx1
Abstract
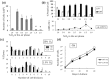
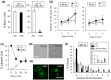
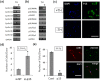
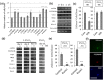
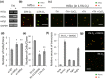
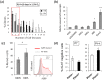
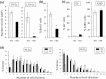
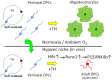
The adult mammalian central nervous system (CNS) contains a population of slowly dividing oligodendrocyte precursor cells (OPCs), i.e., adult OPCs, which supply new oligodendrocytes throughout the life of animal. While adult OPCs develop from rapidly dividing perinatal OPCs, the mechanisms underlying their quiescence remain unknown. Here, we show that perinatal rodent OPCs cultured with thyroid hormone (TH) under hypoxia become quiescent and acquire adult OPCs-like characteristics. The cyclin-dependent kinase inhibitor p15/INK4b plays crucial roles in the TH-dependent cell cycle deceleration in OPCs under hypoxia. Klf9 is a direct target of TH-dependent signaling. Under hypoxic conditions, hypoxia-inducible factors mediates runt-related transcription factor 1 activity to induce G1 arrest in OPCs through enhancing TH-dependent p15/INK4b expression. As adult OPCs display phenotypes of adult somatic stem cells in the CNS, the current results shed light on environmental requirements for the quiescence of adult somatic stem cells during their development from actively proliferating stem/progenitor cells.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Wolswijk G, Noble M. Identification of an adult-specific glial progenitor cell. Development. 1989;105:387–400. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

