Growth Differentiation Factor-15 Deficiency Augments Inflammatory Response and Exacerbates Septic Heart and Renal Injury Induced by Lipopolysaccharide
- PMID: 28432312
- PMCID: PMC5430818
- DOI: 10.1038/s41598-017-00902-5
Growth Differentiation Factor-15 Deficiency Augments Inflammatory Response and Exacerbates Septic Heart and Renal Injury Induced by Lipopolysaccharide
Abstract
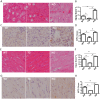
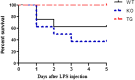
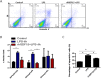
Septic acute kidney injury (AKI) and myocardial dysfunction are leading causes of mortality with no accepted method of therapy. In this study we demonstrate the role of growth differentiating factor 15 (GDF15) in septic AKI and myocardial dysfunction using a murine lipopolysaccharide (LPS)-induced sepsis model and an in vitro cell culture system. Data show that GDF15 deficiency augments inflammatory response and exacerbates renal and cardiac injury induced by LPS, while over-expression of GDF15 protects the kidney and heart from LPS-induced organ dysfunction. Therefore, this study highlights the therapeutic potential of GDF15 in the treatment of endotoxin-induced sepsis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Bhan, C., Dipankar, P., Chakraborty, P. & Sarangi, P.P. Role of cellular events in the pathophysiology of sepsis. Inflammation research: official journal of the European Histamine Research Society … [et al.] (2016). - PubMed
-
- Husak, L. et al. National analysis of sepsis hospitalizations and factors contributing to sepsis in-hospital mortality in Canada. Healthcare quarterly (Toronto, Ont.) 13 Spec No, 35–41 (2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

