The GENDER ATTENTION Observational Study: Gender and Hormonal Status Differences in the Incidence of Adverse Events During Cyclosporine Treatment in Psoriatic Patients
- PMID: 28432647
- PMCID: PMC5487861
- DOI: 10.1007/s12325-017-0526-7
The GENDER ATTENTION Observational Study: Gender and Hormonal Status Differences in the Incidence of Adverse Events During Cyclosporine Treatment in Psoriatic Patients
Abstract
Introduction: Female sex has been shown to be a risk factor for the development of adverse drug reactions; however, this has not been studied for cyclosporine (CsA). The aim of this study was to investigate, in Italian dermatological practice, the influence of gender and menopause and related hormones on the incidence of adverse events (AEs) during CsA treatment in psoriatic patients.
Methods: Multicenter, prospective, observational study conducted from May 2011 to June 2013. Patients with plaque psoriasis, undergoing a new CsA administration course, or about to start it, were enrolled in the outpatient clinics of Italian dermatological centers. During the 2-6 months of study duration, patients had to note all AEs that occurred in a diary that was reviewed by the investigators at the follow-up visit. Sex hormone levels were measured within 7 days from the start date of a menstrual cycle.
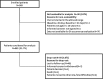
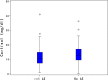
Results: A total of 969 adult psoriatic patients were enrolled in the study, divided into four cohorts: fertile women and corresponding age-matched men; postmenopausal women and corresponding age-matched men. A significant difference in the percentage of patients with AEs was observed between fertile and postmenopausal women, but not between women and age-matched men. AE incidence rate was about 37% higher in fertile women than in age-matched men and about 18% higher in postmenopausal women than in age-matched men, but differences were not statistically significant. Incidence rate ratio of fertile vs. postmenopausal women was 0.67, reaching statistical significance. AEs were mild or moderate in severity in the great majority of patients of all cohorts and postmenopausal women had significantly less grade 1-2 AEs compared to fertile women, but more grade 3-4 AEs. FSH levels were significantly higher in postmenopausal women reporting no AEs, and DHEA sulfate levels were about 10% higher in men with no AEs, compared to those reporting at least one AE. Cortisol levels were slightly though significantly higher in postmenopausal women with no AE.
Conclusions: A better understanding of sex- and hormone-related influences on drug responses may help to improve drug safety and efficacy, by permitting one to tailor pharmacological treatments to individual subjects or defined patient cohorts.
Funding: Novartis Farma S.p.A., Italy.
Keywords: Adverse drug reaction; Cyclosporine; Dermatology; Female; Gender; Psoriasis.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

