Immediate versus delayed postpartum insertion of contraceptive implant for contraception
- PMID: 28432791
- PMCID: PMC6478153
- DOI: 10.1002/14651858.CD011913.pub2
Immediate versus delayed postpartum insertion of contraceptive implant for contraception
Update in
-
Immediate versus delayed postpartum insertion of contraceptive implant and IUD for contraception.Cochrane Database Syst Rev. 2022 Oct 27;10(10):CD011913. doi: 10.1002/14651858.CD011913.pub3. Cochrane Database Syst Rev. 2022. PMID: 36302159 Free PMC article.
Abstract
Background: The spacing of pregnancies has a positive impact on maternal and newborn health. The progestin contraceptive implant, which is a long-acting, reversible method of contraception, has a well-established low failure rate that is compatible with tubal sterilization. The standard provision of contraceptive methods on the first postpartum visit may put some women at risk of unintended pregnancy, either due to loss to follow-up or having sexual intercourse prior to receiving contraception. Therefore, the immediate administration of contraception prior to discharge from the hospital that has high efficacy may improve contraceptive prevalence and prevent unintended pregnancy.
Objectives: To compare the initiation rate, effectiveness, and side effects of immediate versus delayed postpartum insertion of implant for contraception.
Search methods: We searched for eligible studies up to 28 October 2016 in the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, and POPLINE. We examined review articles and contacted investigators. We also checked registers of ongoing clinical trials, citation lists of included studies, key textbooks, grey literature, and previous systematic reviews for potentially relevant studies.
Selection criteria: We sought randomised controlled trials (RCTs) that compared immediate postpartum versus delayed insertion of contraceptive implant for contraception.
Data collection and analysis: Two review authors (JS, YW) independently screened titles and abstracts of the search results, and assessed the full-text articles of potentially relevant studies for inclusion. They extracted data from the included studies, assessed risk of bias, compared results, and resolved disagreements by consulting a third review author (PL or SK). We contacted investigators for additional data, where possible. We computed the Mantel-Haenszel risk ratio (RR) with 95% confidence interval (CI) for binary outcomes and the mean difference (MD) with 95% CI for continuous variables.
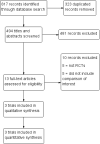
Main results: Three studies that included 410 participants met the inclusion criteria of the review. We did not identify any ongoing trials. Two included studies were at low risk of selection, attrition, and reporting biases, but were at high risk of performance and detection biases due to the inability to blind participants to the intervention. One included study was at high risk of attrition bias. The overall quality of the evidence for each comparison ranged from very low to moderate; the main limitations were risk of bias and imprecision.Initiation rate of contraceptive implants at the first postpartum check-up visit was significantly higher in the immediate insertion group than in the delayed insertion group (RR 1.41, 95% CI 1.28 to 1.55; three studies, 410 participants; moderate quality evidence).There appeared to be little or no difference between the groups in the continuation rate of contraceptive implant used at six months after insertion (RR 1.02, 95% CI 0.93 to 1.11; two studies, 125 participants; low quality evidence) or at 12 months after insertion (RR 1.04; 95% CI 0.81 to 1.34; one study, 64 participants;very low quality evidence)Women who received an immediate postpartum contraceptive implant insertion had a higher mean number of days of abnormal vaginal bleeding within six weeks postpartum (MD 5.80 days, 95% CI 3.79 to 7.81; one study, 215 participants; low quality evidence) and a higher rate of other side effects in the first six weeks after birth (RR 2.06, 95% CI 1.38 to 3.06; one study, 215 participants; low quality evidence) than those who received a delayed postpartum insertion. There appeared to be little or no difference between the groups in heavy, irregular vaginal bleeding or associated severe cramping within 12 months (RR 1.01, 95% CI 0.72 to 1.44, one study, 64 participants;very low quality evidence).It was unclear whether there was any difference between the groups in scores for participant satisfaction on a 0-10 scale (MD -0.40, 95% CI -1.26 to 0.46, low quality evidence), or in rates of unintended pregnancy (RR 1.82, 95% CI 0.38 to 8.71, 1 RCT, 64 women, very low quality evidence) at 12 months, or in rate of breastfeeding rate at six months (RR 2.01, 95% CI 0.72 ro 5.63, 1 RCT, 64 women, very low quality evidence) rate did not differ significantly between the groups.
Authors' conclusions: Evidence from this review indicates that the rate of initiation of contraceptive implant at the first postpartum check-up visit was higher with immediate postpartum insertion than with delayed insertion. There appeared to be little or no difference between the groups in the continuation rate of contraceptive implant use at 6 months. It was unclear whether there was any difference between the groups in continuation of contraceptive use at 12 months or in the unintended pregnancy rate at 12 months.
Conflict of interest statement
Jen Sothornwit: none known
Yuthapong Werawatakul: none known
Srinaree Kaewrudee: none known
Pisake Lumbiganon: none known
Malinee Laopaiboon: none known
Figures





References
References to studies included in this review
Bryant 2016 {published data only}
-
- Bryant AG, Bauer AE, Stuart GS, Levi EE, Zerden ML, Danvers A, Garrett JM. Etonogestrel‐releasing contraceptive implant for postpartum adolescents: a randomized controlled trial. Journal of Pediatric and Adolescent Gynecology 2016 Aug 22 [Epub ahead of print]. [DOI: 10.1016/j.jpag.2016.08.003] - DOI - PubMed
Gurtcheff 2011 {published data only}
-
- Gurtcheff SE, Turok DK, Stoddard G, Murphy PA, Gibson M, Jones KP. Lactogenesis after early postpartum use of the contraceptive implant: a randomized controlled trial. Obstetrics and Gynecology 2011;117(5):1114‐21. - PubMed
Phemister 1995 {published data only}
-
- Phemister DA, Laurent S, Harrison FN Jr. Use of Norplant contraceptive implants in the immediate postpartum period: safety and tolerance. American Journal of Obstetrics and Gynecology 1995;172(1 Pt 1):175‐9. - PubMed
References to studies excluded from this review
Braga 2015 {published data only}
-
- Braga GC, Ferriolli E, Quintana SM, Ferriani RA, Pfrimer K, Vieira CS. Immediate postpartum initiation of etonogestrel‐releasing implant: a randomized controlled trial on breastfeeding impact. Contraception 2015;92(6):536‐42. - PubMed
Brito 2009 {published data only}
-
- Brito MB, Ferriani RA, Quintana SM, Yazlle ME, Silva de Sá MF, Vieira CS. Safety of the etonogestrel‐releasing implant during the immediate postpartum period: a pilot study. Contraception 2009;80(6):519‐26. - PubMed
Brito 2012 {published data only}
-
- Brito MB, Ferriani RA, Meijers JC, Garcia AA, Quintana SM, Silva de Sá MF, et al. Effects of the etonogestrel‐releasing contraceptive implant inserted immediately postpartum on maternal hemostasis: a randomized controlled trial. Thrombosis Research 2012;130(3):355‐60. - PubMed
Gariepy 2015 {published data only}
Ireland 2014 {published data only}
-
- Ireland LD, Goyal V, Raker CA, Murray A, Allen RH. The effect of immediate postpartum compared to delayed postpartum and interval etonogestrel contraceptive implant insertion on removal rates for bleeding. Contraception 2014;90(3):253–8. - PubMed
Pentickly 2013 {published data only}
-
- Pentickly S, Ratcliffe SJ, Schreiber C. The impact of progestin‐only contraceptives on postpartum weight loss (POPP): a year‐long randomized controlled study. Contraception 2013;88(3):434.
Shabaan 1985 {published data only}
-
- Shaaban MM, Salem HT, Abdullah KA. Influence of levonorgestrel contraceptive implants, NORPLANT, initiated early postpartum upon lactation and infant growth. Contraception 1985;32(6):623‐35. - PubMed
Taneepanichkul 2001 {published data only}
-
- Taneepanichskul S, Tanprasertkul C. Use of Norplant implants in the immediate postpartum period among asymptomatic HIV‐1‐positive mothers. Contraception 2001;64(1):39‐41. - PubMed
Tocce 2012 {published data only}
-
- Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference?. American Journal of Obstetrics and Gynecology 2012;206(6):481.e1‐7. - PubMed
Wilson 2014 {published data only}
-
- Wilson S, Tennant C, Sammel MD, Schreiber C. Immediate postpartum etonogestrel implant: a contraception option with long‐term continuation. Contraception 2014;90(3):259‐64. - PubMed
Additional references
ACOG 2011
-
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 121: Long‐acting reversible contraception: Implants and intrauterine devices. Obstetrics and Gynecology 2011;118(1):184‐96. - PubMed
ACOG 2012
-
- Committee on Adolescent Health Care Long‐Acting Reversible Contraception Working Group, The American College of Obstetricians and Gynecologists. Committee opinion no. 539: adolescents and long‐acting reversible contraception: implants and intrauterine devices. Obstetrics and Gynecology 2012;120(4):983‐8. - PubMed
Baldwin 2013
Campbell 2000
-
- Campbell MJ. Cluster randomised trials in general (family) practice research. Statistical Methods in Medical Research 2000;9(2):81‐94. - PubMed
Chaovisitsaree 2012
-
- Chaovisitsaree S, Noi‐um S, Kietpeerakool C. Review of postpartum contraceptive practices at Chiang Mai University Hospital: implications for improving quality of service. Medical Principles and Practice 2012;21(2):145‐9. - PubMed
Deeks 2001
-
- Deeks J, Altman D, Bradburn M. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐Analysis in Context. 2nd Edition. London: BMJ Publication Group, 2001.
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
EndNote 2015 [Computer program]
-
- Thomas Reuters. EndNote. Version X7. New York: Thomas Reuters, 2015.
Finer 2011
-
- Finer LB, Kost K. Unintended pregnancy rates at the state level. Perspectives on Sexual and Reproductive Health 2011;43(2):78‐87. - PubMed
Fraser 1995
-
- Fraser AM, Brockert JE, Ward RH. Association of young maternal age with adverse reproductive outcomes. New England Journal of Medicine 1995;332(17):1113‐7. - PubMed
GRADEproGDT 2014 [Computer program]
-
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 11 November 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Higgins 2011
-
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Liberati 2009
-
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [DOI: 10.1371/journal.pmed.1000100] - DOI - PMC - PubMed
Lopez 2015
Moore 2015
-
- Moore Z, Pfitzer A, Gubin R, Charurat E, Elliott L, Croft T. Missed opportunities for family planning: an analysis of pregnancy risk and contraceptive method use among postpartum women in 21 low‐ and middle‐income countries. Contraception 2015;92(1):31‐9. [DOI: 10.1016/j.contraception.2015.03.007] - DOI - PubMed
Nkwabong 2015
-
- Nkwabong E, Ilue EE, Bisong CE. Factors associated with poor attendance at the postpartum clinic six weeks after delivery in Cameroon. International Journal of Gynaecology and Obstetrics 2015;129(3):248‐50. - PubMed
Peipert 2011
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Singh 2010
-
- Singh S, Sedgh G, Hussain R. Unintended pregnancy: worldwide levels, trends, and outcomes. Studies in Family Planning 2010;41(4):241‐50. - PubMed
Speroff 2008
-
- Speroff L, Mishell DR Jr. The postpartum visit: it's time for a change in order to optimally initiate contraception. Contraception 2008;78(2):90‐8. - PubMed
Sterne 2011
-
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. - PubMed
Thiel de Bocanegra 2013
-
- Thiel de Bocanegra H, Chang R, Menz M, Howell M, Darney P. Postpartum contraception in publicly‐funded programs and interpregnancy intervals. Obstetrics and Gynecology 2013;122(2 Pt 1):296‐303. - PubMed
Ukoumunne 1999
-
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JA, Burney PG. Methods for evaluating area‐wide and organisation‐based interventions in health and health care: a systematic review. Health Technology Assessment 1999;3(5):iii‐92. - PubMed
UN 2013
-
- United Nations (UN), Department of Economic and Social Affairs, Population Division. World contraceptive patterns 2013. http://www.un.org/en/development/desa/population/publications/family/con... (accessed 28 October 2016).
Whaley 2015
-
- Whaley N, Burke A. Contraception in the postpartum period: immediate options for long‐acting success. Women's Health (London, England) 2015;11(2):97‐9. - PubMed
WHO 2015
-
- Department of Reproductive Health, World Health Organization. Medical eligibility criteria for contraceptive use. Fifth edition 2015. http://apps.who.int/iris/bitstream/10665/172915/1/WHO_RHR_15.07_eng.pdf?... (accessed 10 September 2015).
Wilson 2011
-
- Wilson EK, Samandari G, Koo HP, Tucker C. Adolescent mothers' postpartum contraceptive use: a qualitative study. Perspectives on Sexual and Reproductive Health 2011;43(4):230‐7. - PubMed
References to other published versions of this review
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

