Maternal low-protein diet decreases brain-derived neurotrophic factor expression in the brains of the neonatal rat offspring
- PMID: 28432877
- PMCID: PMC5466833
- DOI: 10.1016/j.jnutbio.2017.03.005
Maternal low-protein diet decreases brain-derived neurotrophic factor expression in the brains of the neonatal rat offspring
Abstract
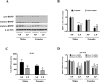
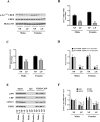
Prenatal exposure to a maternal low-protein (LP) diet has been known to cause cognitive impairment, learning and memory deficits. However, the underlying mechanisms have not been identified. Herein, we demonstrate that a maternal LP diet causes, in the brains of the neonatal rat offspring, an attenuation in the basal expression of the brain-derived neurotrophic factor (BDNF), a neurotrophin indispensable for learning and memory. Female rats were fed either a 20% normal protein (NP) diet or an 8% LP 3 weeks before breeding and during the gestation period. Maternal LP diet caused a significant reduction in the Bdnf expression in the brains of the neonatal rats. We further found that the maternal LP diet reduced the activation of the cAMP/protein kinase A/cAMP response element binding protein (CREB) signaling pathway. This reduction was associated with a significant decrease in CREB binding to the Bdnf promoters. We also show that prenatal exposure to the maternal LP diet results in an inactive or repressed exon I and exon IV promoter of the Bdnf gene in the brain, as evidenced by fluxes in signatory hallmarks in the enrichment of acetylated and trimethylated histones in the nucleosomes that envelop the exon I and exon IV promoters, causing the Bdnf gene to be refractory to transactivation. Our study is the first to determine the impact of a maternal LP diet on the basal expression of BDNF in the brains of the neonatal rats exposed prenatally to an LP diet.
Keywords: Brain-derived neurotrophic factor; Cyclic adenosine monophosphate; Histone acetylation; Histone methylation; Maternal low-protein diet; cAMP response element binding protein.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Involvement of an upstream stimulatory factor as well as cAMP-responsive element-binding protein in the activation of brain-derived neurotrophic factor gene promoter I.J Biol Chem. 2002 Sep 27;277(39):35920-31. doi: 10.1074/jbc.M204784200. Epub 2002 Jul 11. J Biol Chem. 2002. PMID: 12114522
-
cAMP/PKA-CREB-BDNF signaling pathway in hippocampus mediates cyclooxygenase 2-induced learning/memory deficits of rats subjected to chronic unpredictable mild stress.Oncotarget. 2017 May 30;8(22):35558-35572. doi: 10.18632/oncotarget.16009. Oncotarget. 2017. PMID: 28415673 Free PMC article.
-
Effects of maternal separation and antidepressant drug on epigenetic regulation of the brain-derived neurotrophic factor exon I promoter in the adult rat hippocampus.Psychiatry Clin Neurosci. 2018 Apr;72(4):255-265. doi: 10.1111/pcn.12609. Epub 2017 Nov 14. Psychiatry Clin Neurosci. 2018. PMID: 28990703
-
HDAC inhibition prevents hypobaric hypoxia-induced spatial memory impairment through PΙ3K/GSK3β/CREB pathway.J Cell Physiol. 2021 Sep;236(9):6754-6771. doi: 10.1002/jcp.30337. Epub 2021 Mar 31. J Cell Physiol. 2021. PMID: 33788269
-
Effect of XingPiJieYu decoction on spatial learning and memory and cAMP-PKA-CREB-BDNF pathway in rat model of depression through chronic unpredictable stress.BMC Complement Altern Med. 2017 Jan 24;17(1):73. doi: 10.1186/s12906-016-1543-9. BMC Complement Altern Med. 2017. PMID: 28118829 Free PMC article.
Cited by
-
The effect of enriched environment across ages: A study of anhedonia and BDNF gene induction.Genes Brain Behav. 2018 Nov;17(8):e12485. doi: 10.1111/gbb.12485. Epub 2018 May 31. Genes Brain Behav. 2018. PMID: 29717802 Free PMC article.
-
Microglial Dystrophy and Spatial Learning Impairments Following Exposure to Multiple Early-life Stressors in Rats.Ann Neurosci. 2025 Jul 18:09727531251324716. doi: 10.1177/09727531251324716. Online ahead of print. Ann Neurosci. 2025. PMID: 40686539 Free PMC article.
-
Maternal low protein diet and fetal programming of lean type 2 diabetes.World J Diabetes. 2022 Mar 15;13(3):185-202. doi: 10.4239/wjd.v13.i3.185. World J Diabetes. 2022. PMID: 35432755 Free PMC article. Review.
-
Intrauterine Growth Restriction Alters Postnatal Hippocampal Dentate Gyrus Neuron and Microglia Morphology and Cytokine/Chemokine Milieu in Mice.Life (Basel). 2024 Dec 9;14(12):1627. doi: 10.3390/life14121627. Life (Basel). 2024. PMID: 39768335 Free PMC article.
-
A maternal low-protein diet results in sex-specific differences in synaptophysin expression and milk fatty acid profiles in neonatal rats.J Nutr Sci. 2024 Oct 14;13:e64. doi: 10.1017/jns.2024.46. eCollection 2024. J Nutr Sci. 2024. PMID: 39469193 Free PMC article.
References
-
- Flores O, Perez H, Valladares L, Morgan C, Gatica A, Burgos H, et al. Hidden prenatal malnutrition in the rat: role of beta(1)-adrenoceptors on synaptic plasticity in the frontal cortex. J Neurochem. 2011;119:314–23. - PubMed
-
- Morgane PJ, Austin-LaFrance R, Bronzino J, Tonkiss J, Diaz-Cintra S, Cintra L, et al. Prenatal malnutrition and development of the brain. Neurosci Biobehav Rev. 1993;17:91–128. - PubMed
-
- Morgane PJ, Mokler DJ, Galler JR. Effects of prenatal protein malnutrition on the hippocampal formation. Neurosci Biobehav Rev. 2002;26:471–83. - PubMed
-
- Tonkiss J, Galler J, Morgane PJ, Bronzino JD, Austin-LaFrance RJ. Prenatal protein malnutrition and postnatal brain function. Ann N Y Acad Sci. 1993;678:215–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

