DNA Methylation program in normal and alcohol-induced thinning cortex
- PMID: 28433420
- PMCID: PMC5664948
- DOI: 10.1016/j.alcohol.2017.01.006
DNA Methylation program in normal and alcohol-induced thinning cortex
Abstract
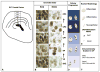
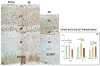
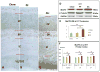
While cerebral underdevelopment is a hallmark of fetal alcohol spectrum disorders (FASD), the mechanism(s) guiding the broad cortical neurodevelopmental deficits are not clear. DNA methylation is known to regulate early development and tissue specification through gene regulation. Here, we examined DNA methylation in the onset of alcohol-induced cortical thinning in a mouse model of FASD. C57BL/6 (B6) mice were administered a 4% alcohol (v/v) liquid diet from embryonic (E) days 7-16, and their embryos were harvested at E17, along with isocaloric liquid diet and lab chow controls. Cortical neuroanatomy, neural phenotypes, and epigenetic markers of methylation were assessed using immunohistochemistry, Western blot, and methyl-DNA assays. We report that cortical thickness, neuroepithelial proliferation, and neuronal migration and maturity were found to be deterred by alcohol at E17. Simultaneously, DNA methylation, including 5-methylcytosine (5mC) and 5-hydroxcylmethylcytosine (5hmC), which progresses as an intrinsic program guiding normal embryonic cortical development, was severely affected by in utero alcohol exposure. The intricate relationship between cortical thinning and this DNA methylation program disruption is detailed and illustrated. DNA methylation, dynamic across the multiple cortical layers during the late embryonic stage, is highly disrupted by fetal alcohol exposure; this disruption occurs in tandem with characteristic developmental abnormalities, ranging from structural to molecular. Finally, our findings point to a significant question for future exploration: whether epigenetics guides neurodevelopment or whether developmental conditions dictate epigenetic dynamics in the context of alcohol-induced cortical teratogenesis.
Keywords: Epigenetics; Fetal Alcohol Spectrum Disorders (FASD); Neurodevelopmental deficit; Neuroepigenetics; Neurogenesis.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures






Similar articles
-
Epigenetic mediators and consequences of excessive alcohol consumption.Alcohol. 2017 May;60:1-6. doi: 10.1016/j.alcohol.2017.02.357. Epub 2017 Mar 11. Alcohol. 2017. PMID: 28395929 Free PMC article. Review. No abstract available.
-
Long-term alterations to DNA methylation as a biomarker of prenatal alcohol exposure: From mouse models to human children with fetal alcohol spectrum disorders.Alcohol. 2017 May;60:67-75. doi: 10.1016/j.alcohol.2016.11.009. Epub 2016 Nov 22. Alcohol. 2017. PMID: 28187949 Review.
-
DNA methylation-independent growth restriction and altered developmental programming in a mouse model of preconception male alcohol exposure.Epigenetics. 2017;12(10):841-853. doi: 10.1080/15592294.2017.1363952. Epub 2017 Dec 7. Epigenetics. 2017. PMID: 28816587 Free PMC article.
-
Changes to histone modifications following prenatal alcohol exposure: An emerging picture.Alcohol. 2017 May;60:41-52. doi: 10.1016/j.alcohol.2017.01.005. Epub 2017 Feb 4. Alcohol. 2017. PMID: 28431792 Review.
-
Pre-implantation alcohol exposure induces lasting sex-specific DNA methylation programming errors in the developing forebrain.Clin Epigenetics. 2021 Aug 23;13(1):164. doi: 10.1186/s13148-021-01151-0. Clin Epigenetics. 2021. PMID: 34425890 Free PMC article.
Cited by
-
Epigenetic mediators and consequences of excessive alcohol consumption.Alcohol. 2017 May;60:1-6. doi: 10.1016/j.alcohol.2017.02.357. Epub 2017 Mar 11. Alcohol. 2017. PMID: 28395929 Free PMC article. Review. No abstract available.
-
An overview of current advances in perinatal alcohol exposure and pathogenesis of fetal alcohol spectrum disorders.J Neurodev Disord. 2024 Apr 20;16(1):20. doi: 10.1186/s11689-024-09537-w. J Neurodev Disord. 2024. PMID: 38643092 Free PMC article. Review.
-
Prenatal Alcohol Exposure: Profiling Developmental DNA Methylation Patterns in Central and Peripheral Tissues.Front Genet. 2018 Dec 4;9:610. doi: 10.3389/fgene.2018.00610. eCollection 2018. Front Genet. 2018. PMID: 30568673 Free PMC article.
-
Hippocampal DNA Methylation in a Mouse Model of Fetal Alcohol Spectrum Disorder That Includes Maternal Separation Stress Only Partially Explains Changes in Gene Expression.Front Genet. 2020 Feb 27;11:70. doi: 10.3389/fgene.2020.00070. eCollection 2020. Front Genet. 2020. PMID: 32174962 Free PMC article.
-
Environmental Epigenetics and Genome Flexibility: Focus on 5-Hydroxymethylcytosine.Int J Mol Sci. 2020 May 2;21(9):3223. doi: 10.3390/ijms21093223. Int J Mol Sci. 2020. PMID: 32370155 Free PMC article. Review.
References
-
- Angevine JB, Jr, Sidman RL. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature. 1961;192:766–768. - PubMed
-
- Aronne MP, Evrard SG, Mirochnic S, Brusco A. Prenatal ethanol exposure reduces the expression of the transcriptional factor Pax6 in the developing rat brain. Annals of the New York Academy of Sciences. 2008;1139:478–498. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

