Regulation of human nucleus pulposus cells by peptide-coupled substrates
- PMID: 28433788
- PMCID: PMC5536110
- DOI: 10.1016/j.actbio.2017.04.019
Regulation of human nucleus pulposus cells by peptide-coupled substrates
Abstract
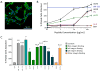
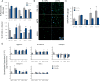
Nucleus pulposus (NP) cells are derived from the notochord and differ from neighboring cells of the intervertebral disc in phenotypic marker expression and morphology. Adult human NP cells lose this phenotype and morphology with age in a pattern that contributes to progressive disc degeneration and pathology. Select laminin-mimetic peptide ligands and substrate stiffnesses were examined for their ability to regulate human NP cell phenotype and biosynthesis through the expression of NP-specific markers aggrecan, N-cadherin, collagen types I and II, and GLUT1. Peptide-conjugated substrates demonstrated an ability to promote expression of healthy NP-specific markers, as well as increased biosynthetic activity. We show an ability to re-express markers of the juvenile NP cell and morphology through control of peptide presentation and stiffness on well-characterized polyacrylamide substrates. NP cells cultured on surfaces conjugated with α3 integrin receptor peptides P4 and P678, and on α2, α5, α6, β1 integrin-recognizing peptide AG10, show increased expression of aggrecan, N-cadherin, and types I and II collagen, suggesting a healthier, more juvenile-like phenotype. Multi-cell cluster formation was also observed to be more prominent on peptide-conjugated substrates. These findings indicate a critical role for cell-matrix interactions with specific ECM-mimetic peptides in supporting and maintaining a healthy NP cell phenotype and bioactivity.
Statement of significance: NP cells reside in a laminin-rich environment that deteriorates with age, including a loss of water content and changes in the extracellular matrix (ECM) structure that may lead to the development of a degenerated IVD. There is great interest in methods to re-express healthy, biosynthetically active NP cells using laminin-derived biomimetic peptides toward the goal of using autologous cell sources for tissue regeneration. Here, we describe a novel study utilizing several laminin mimetic peptides conjugated to polyacrylamide gels that are able to support an immature, healthy NP phenotype after culture on "soft" peptide gels. These findings can support future studies in tissue regeneration where cells may be directed to a desired regenerative phenotype using niche-specific ECM peptides.
Keywords: Cell-matrix interactions; Extracellular matrix; Hydrogel; Mechanobiology; Polyacrylamide; Spine.
Copyright © 2017 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures


References
-
- Sakai D, Nakamura Y, Nakai T, Mishima T, Kato S, Grad S, Alini M, Risbud MV, Chan D, Cheah KS, Yamamura K, Masuda K, Okano H, Ando K, Mochida J. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nature communications. 2012;3:1264. - PMC - PubMed
-
- Tran CM, Schoepflin ZR, Markova DZ, Kepler CK, Anderson DG, Shapiro IM, Risbud MV. CCN2 suppresses catabolic effects of interleukin-1beta through alpha5beta1 and alphaVbeta3 integrins in nucleus pulposus cells: implications in intervertebral disc degeneration. The Journal of biological chemistry. 2014;289(11):7374–87. - PMC - PubMed
-
- Risbud MV, Guttapalli A, Albert TJ, Shapiro IM. Hypoxia activates MAPK activity in rat nucleus pulposus cells: regulation of integrin expression and cell survival. Spine (Phila Pa 1976) 2005;30(22):2503–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

