Role of environmental stressors in determining the developmental outcome of neonatal anesthesia
- PMID: 28433802
- PMCID: PMC5492971
- DOI: 10.1016/j.psyneuen.2017.04.001
Role of environmental stressors in determining the developmental outcome of neonatal anesthesia
Abstract
Background: The majority of studies evaluating neurocognition in humans who had procedures under anesthesia early in life found long-term deficits even though the typical anesthesia duration normalized to the human life span is much shorter than that shown to induce developmental abnormalities in rodents. Therefore, we studied whether subsequent environmental stressors contribute to deficiencies programmed by a brief neonatal etomidate exposure.
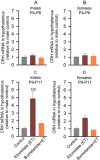
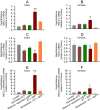
Methods: Postnatal days (P) 4, 5, or 6, Sprague-Dawley rats, pretreated with vehicle or the Na+-K+-2Cl- (NKCC1) inhibitor, bumetanide, received two injections of etomidate resulting in anesthesia for 2h. To simulate stress after anesthesia, the animals were exposed to a single maternal separation for 3h at P10. 3-7days after exposure to etomidate the rats had increased hypothalamic NKCC1 mRNA and corticotropin releasing hormone (CRH) mRNA and decreased K+-2Cl- (KCC2) mRNA levels with greater changes in males. In rats neonatally exposed to both etomidate and maternal separation, these abnormalities persisted into adulthood. These animals also exhibited extended corticosterone responses to restraint stress with increases in total plasma corticosterone more robust in males, as well as behavioral abnormalities. Pretreatment with the NKCC1 inhibitor ameliorated most of these effects.
Conclusions: Post-anesthesia stressors may exacerbate/unmask neurodevelopmental abnormalities even after a relatively short anesthetic with etomidate, leading to dysregulated stress response systems and neurobehavioral deficiencies in adulthood. Amelioration by bumetanide suggests a mechanistic role for etomidate-enhanced gamma-aminobutyric acid type A receptor-mediated depolarization in initiating long-lasting alterations in gene expression that are further potentiated by subsequent maternal separation.
Keywords: Behavior; Developing brain; Environmental factor; Etomidate; Maternal separation; Stress.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

