Topiramate monotherapy for juvenile myoclonic epilepsy
- PMID: 28434203
- PMCID: PMC6478307
- DOI: 10.1002/14651858.CD010008.pub3
Topiramate monotherapy for juvenile myoclonic epilepsy
Update in
-
Topiramate for juvenile myoclonic epilepsy.Cochrane Database Syst Rev. 2019 Jan 28;1(1):CD010008. doi: 10.1002/14651858.CD010008.pub4. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2021 Nov 24;11:CD010008. doi: 10.1002/14651858.CD010008.pub5. PMID: 30687937 Free PMC article. Updated.
Abstract
Background: Topiramate is a newer broad-spectrum antiepileptic drug (AED). Some studies have shown the benefits of topiramate monotherapy in the treatment of juvenile myoclonic epilepsy (JME). However, there are no current systematic reviews to determine the efficacy and tolerability of topiramate monotherapy in people with JME. This is an updated version of the original Cochrane Review published in Issue 12, 2015.
Objectives: To evaluate the efficacy and tolerability of topiramate monotherapy in the treatment of JME.
Search methods: For the latest update, on 21 February 2017 we searched Cochrane Epilepsy's Specialized Register, CENTRAL, MEDLINE, and ClinicalTrials.gov. We also searched ongoing trials registers, reference lists and relevant conference proceedings, and contacted study authors and pharmaceutical companies.
Selection criteria: We included randomized controlled trials (RCTs) investigating topiramate monotherapy versus placebo or other AED treatment for people with JME, with the outcomes of proportion of responders or experiencing adverse events (AEs).
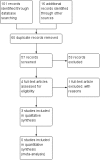
Data collection and analysis: Two review authors independently screened the titles and abstracts of identified records, selected studies for inclusion, extracted data, cross-checked the data for accuracy and assessed the methodological quality. We performed no meta-analyses due to the limited available data.
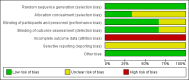
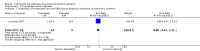
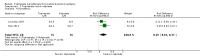
Main results: We included three studies with 83 participants. For efficacy, a greater proportion of participants in the topiramate group had a 50% or more reduction in primarily generalized tonic-clonic seizures (PGTCS) compared with participants in the placebo group. There were no significant differences between topiramate versus valproate in participants responding with a 50% or more reduction in myoclonic seizures or in PGTCS or seizure-free. Concerning tolerability, we ranked AEDs associated with topiramate as moderate-to-severe, while we ranked 59% of AEDs linked to valproate as severe complaints. Moreover, systemic toxicity scores were higher in the valproate group than the topiramate group. We judged the quality of the evidence from the studies to be very low.
Authors' conclusions: Since the last version of this review we found no new studies. This review does not provide sufficient evidence to support topiramate for the treatment of people with JME. Based on the current limited available data, topiramate seems to be better tolerated than valproate, but there were no more benefits of efficacy in topiramate compared with valproate. In the future, well-designed, double-blind RCTs with large samples are required to test the efficacy and tolerability of topiramate in people with JME.
Conflict of interest statement
Jia Liu; none known Lu‐Ning Wang: none known Yu‐Ping Wang: none known
Figures

























Update of
-
Topiramate monotherapy for juvenile myoclonic epilepsy.Cochrane Database Syst Rev. 2015 Dec 23;(12):CD010008. doi: 10.1002/14651858.CD010008.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2017 Apr 23;4:CD010008. doi: 10.1002/14651858.CD010008.pub3. PMID: 26695884 Updated.
Similar articles
-
Topiramate for juvenile myoclonic epilepsy.Cochrane Database Syst Rev. 2021 Nov 24;11(11):CD010008. doi: 10.1002/14651858.CD010008.pub5. Cochrane Database Syst Rev. 2021. PMID: 34817852 Free PMC article.
-
Topiramate monotherapy for juvenile myoclonic epilepsy.Cochrane Database Syst Rev. 2015 Dec 23;(12):CD010008. doi: 10.1002/14651858.CD010008.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2017 Apr 23;4:CD010008. doi: 10.1002/14651858.CD010008.pub3. PMID: 26695884 Updated.
-
Topiramate versus carbamazepine monotherapy for epilepsy: an individual participant data review.Cochrane Database Syst Rev. 2016 Dec 6;12(12):CD012065. doi: 10.1002/14651858.CD012065.pub2. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2019 Jun 24;6:CD012065. doi: 10.1002/14651858.CD012065.pub3. PMID: 27922722 Free PMC article. Updated.
-
Antiepileptic drug monotherapy for epilepsy: a network meta-analysis of individual participant data.Cochrane Database Syst Rev. 2017 Dec 15;12(12):CD011412. doi: 10.1002/14651858.CD011412.pub3. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2022 Apr 1;4:CD011412. doi: 10.1002/14651858.CD011412.pub4. PMID: 29243813 Free PMC article. Updated.
-
Antiepileptic drug monotherapy for epilepsy: a network meta-analysis of individual participant data.Cochrane Database Syst Rev. 2017 Jun 29;6(6):CD011412. doi: 10.1002/14651858.CD011412.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2017 Dec 15;12:CD011412. doi: 10.1002/14651858.CD011412.pub3. PMID: 28661008 Free PMC article. Updated.
Cited by
-
Topiramate for juvenile myoclonic epilepsy.Cochrane Database Syst Rev. 2019 Jan 28;1(1):CD010008. doi: 10.1002/14651858.CD010008.pub4. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2021 Nov 24;11:CD010008. doi: 10.1002/14651858.CD010008.pub5. PMID: 30687937 Free PMC article. Updated.
-
Topiramate for juvenile myoclonic epilepsy.Cochrane Database Syst Rev. 2021 Nov 24;11(11):CD010008. doi: 10.1002/14651858.CD010008.pub5. Cochrane Database Syst Rev. 2021. PMID: 34817852 Free PMC article.
References
References to studies included in this review
-
- Biton V, Bourgeois BF, YTC/YTCE Study Investigators. Topiramate in patients with juvenile myoclonic epilepsy. Archives of Neurology 2005;62:1705‐8. - PubMed
-
- Levisohn PM, Holland KD. Topiramate or valproate in patients with juvenile myoclonic epilepsy: a randomized open‐label comparison. Epilepsy & Behavior 2007;10:547‐52. - PubMed
-
- Park KM, Kim SH, Nho SK, Shin KJ, Park J, Ha SY, et al. A randomized open‐label observational study to compare the efficacy and tolerability between topiramate and valproate in juvenile myoclonic epilepsy. Journal of Clinical Neuroscience 2013;20:1079‐82. - PubMed
References to studies excluded from this review
-
- Sousa Pda S, Araújo Filho GM, Garzon E, Sakamoto AC, Yacubian EM. Topiramate for the treatment of juvenile myoclonic epilepsy. Arquivos de Neuro‐psiquiatria 2005;63:733‐7. - PubMed
Additional references
-
- Alfradique I, Vasconcelos MM. Juvenile myoclonic epilepsy. Arquivos de Neuro‐psiquiatria 2007;65:1266‐71. - PubMed
-
- Biton V, Montouris GD, Ritter F, Riviello JJ, Reife R, Lim P, et al. A randomized, placebo‐controlled study of topiramate in primary generalized tonic‐clonic seizures. Neurology 1999;52:1330‐7. - PubMed
-
- Calleja S, Salas‐Puig J, Ribacoba R, Lahoz CH. Evolution of juvenile myoclonic epilepsy treated from the outset with sodium valproate. Seizure 2001;10:424‐7. - PubMed
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
-
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. - PubMed
References to other published versions of this review
-
- Liu J, Wang LN. Topiramate monotherapy for juvenile myoclonic epilepsy. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD010008] - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

