Stem Cell Lineage Infidelity Drives Wound Repair and Cancer
- PMID: 28434617
- PMCID: PMC5510746
- DOI: 10.1016/j.cell.2017.03.042
Stem Cell Lineage Infidelity Drives Wound Repair and Cancer
Abstract
Tissue stem cells contribute to tissue regeneration and wound repair through cellular programs that can be hijacked by cancer cells. Here, we investigate such a phenomenon in skin, where during homeostasis, stem cells of the epidermis and hair follicle fuel their respective tissues. We find that breakdown of stem cell lineage confinement-granting privileges associated with both fates-is not only hallmark but also functional in cancer development. We show that lineage plasticity is critical in wound repair, where it operates transiently to redirect fates. Investigating mechanism, we discover that irrespective of cellular origin, lineage infidelity occurs in wounding when stress-responsive enhancers become activated and override homeostatic enhancers that govern lineage specificity. In cancer, stress-responsive transcription factor levels rise, causing lineage commanders to reach excess. When lineage and stress factors collaborate, they activate oncogenic enhancers that distinguish cancers from wounds.
Keywords: cancer; epigenetics; lineage infidelity; regeneration; skin; stem cells; stress response; super-enhancers; transcriptional regulation; wound repair.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures


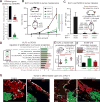



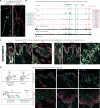
Comment in
-
Don't Stop Re-healin'! Cancer as an Ongoing Stem Cell Affair.Cell. 2017 May 4;169(4):563-565. doi: 10.1016/j.cell.2017.04.030. Cell. 2017. PMID: 28475887
References
-
- Antsiferova M, Werner S. The bright and the dark sides of activin in wound healing and cancer. J Cell Sci. 2012;125:3929–3937. - PubMed
-
- Arwert EN, Hoste E, Watt FM. Epithelial stem cells, wound healing and cancer. Nat Rev Cancer. 2012;12:170–180. - PubMed
-
- Balmain A, Yuspa SH. Milestones in skin carcinogenesis: the biology of multistage carcinogenesis. J Invest Dermatol. 2014;134:E2–7. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

