Apolipoprotein E, Receptors, and Modulation of Alzheimer's Disease
- PMID: 28434655
- PMCID: PMC5599322
- DOI: 10.1016/j.biopsych.2017.03.003
Apolipoprotein E, Receptors, and Modulation of Alzheimer's Disease
Abstract
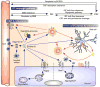
Apolipoprotein E (apoE) is a lipid carrier in both the peripheral and the central nervous systems. Lipid-loaded apoE lipoprotein particles bind to several cell surface receptors to support membrane homeostasis and injury repair in the brain. Considering prevalence and relative risk magnitude, the ε4 allele of the APOE gene is the strongest genetic risk factor for late-onset Alzheimer's disease (AD). ApoE4 contributes to AD pathogenesis by modulating multiple pathways, including but not limited to the metabolism, aggregation, and toxicity of amyloid-β peptide, tauopathy, synaptic plasticity, lipid transport, glucose metabolism, mitochondrial function, vascular integrity, and neuroinflammation. Emerging knowledge on apoE-related pathways in the pathophysiology of AD presents new opportunities for AD therapy. We describe the biochemical and biological features of apoE and apoE receptors in the central nervous system. We also discuss the evidence and mechanisms addressing differential effects of apoE isoforms and the role of apoE receptors in AD pathogenesis, with a particular emphasis on the clinical and preclinical studies related to amyloid-β pathology. Finally, we summarize the current strategies of AD therapy targeting apoE, and postulate that effective strategies require an apoE isoform-specific approach.
Keywords: Alzheimer’s disease; Amyloid-β; Apolipoprotein E; Low-density lipoprotein receptor family; Synaptic plasticity; Tauopathy.
Copyright © 2017 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors report no biomedical financial interests or potential conflicts of interest.
Figures
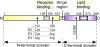

References
-
- Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368:387–403. - PubMed
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
-
- Reger MA, Watson GS, Frey WH, 2nd, Baker LD, Cholerton B, Keeling ML, et al. Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiol Aging. 2006;27:451–458. - PubMed
-
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

