Reduced-dose radiotherapy for human papillomavirus-associated squamous-cell carcinoma of the oropharynx: a single-arm, phase 2 study
- PMID: 28434660
- PMCID: PMC6488353
- DOI: 10.1016/S1470-2045(17)30246-2
Reduced-dose radiotherapy for human papillomavirus-associated squamous-cell carcinoma of the oropharynx: a single-arm, phase 2 study
Abstract
Background: Head and neck cancers positive for human papillomavirus (HPV) are exquisitely radiosensitive. We investigated whether chemoradiotherapy with reduced-dose radiation would maintain survival outcomes while improving tolerability for patients with HPV-positive oropharyngeal carcinoma.
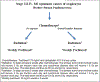
Methods: We did a single-arm, phase 2 trial at two academic hospitals in the USA, enrolling patients with newly diagnosed, biopsy-proven stage III or IV squamous-cell carcinoma of the oropharynx, positive for HPV by p16 testing, and with Zubrod performance status scores of 0 or 1. Patients received two cycles of induction chemotherapy with 175 mg/m2 paclitaxel and carboplatin (target area under the curve of 6) given 21 days apart, followed by intensity-modulated radiotherapy with daily image guidance plus 30 mg/m2 paclitaxel per week concomitantly. Complete or partial responders to induction chemotherapy received 54 Gy in 27 fractions, and those with less than partial or no responses received 60 Gy in 30 fractions. The primary endpoint was progression-free survival at 2 years, assessed in all eligible patients who completed protocol treatment. This study is registered with ClinicalTrials.gov, numbers NCT02048020 and NCT01716195.
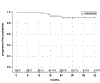
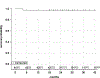
Findings: Between Oct 4, 2012, and March 3, 2015, 45 patients were enrolled with a median age of 60 years (IQR 54-67). One patient did not receive treatment and 44 were included in the analysis. 24 (55%) patients with complete or partial responses to induction chemotherapy received 54 Gy radiation, and 20 (45%) with less than partial responses received 60 Gy. Median follow-up was 30 months (IQR 26-37). Three (7%) patients had locoregional recurrence and one (2%) had distant metastasis; 2-year progression-free survival was 92% (95% CI 77-97). 26 (39%) of 44 patients had grade 3 adverse events, but no grade 4 events were reported. The most common grade 3 events during induction chemotherapy were leucopenia (17 [39%]) and neutropenia (five [11%]), and during chemoradiotherapy were dysphagia (four [9%]) and mucositis (four [9%]). One (2%) of 44 patients was dependent on a gastrostomy tube at 3 months and none was dependent 6 months after treatment.
Interpretation: Chemoradiotherapy with radiation doses reduced by 15-20% was associated with high progression-free survival and an improved toxicity profile compared with historical regimens using standard doses. Radiotherapy de-escalation has the potential to improve the therapeutic ratio and long-term function for these patients.
Funding: University of California.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures
Comment in
-
HPV-related oropharyngeal carcinoma de-escalation protocols.Lancet Oncol. 2017 Jun;18(6):704-705. doi: 10.1016/S1470-2045(17)30250-4. Epub 2017 Apr 20. Lancet Oncol. 2017. PMID: 28434659 No abstract available.
-
Prospective radiotherapy for patients with oropharyngeal carcinoma.Lancet Oncol. 2017 Aug;18(8):e425. doi: 10.1016/S1470-2045(17)30412-6. Epub 2017 Jul 26. Lancet Oncol. 2017. PMID: 28759372 No abstract available.
-
Prospective radiotherapy for patients with oropharyngeal carcinoma - Authors' reply.Lancet Oncol. 2017 Aug;18(8):e426. doi: 10.1016/S1470-2045(17)30534-X. Epub 2017 Jul 26. Lancet Oncol. 2017. PMID: 28759373 No abstract available.
-
Two Sides of the Same Coin: Head and Neck Cancer Treatment De-Intensification and Intensification with Induction Chemotherapy.Int J Radiat Oncol Biol Phys. 2018 Sep 1;102(1):1-4. doi: 10.1016/j.ijrobp.2018.04.002. Epub 2018 Aug 8. Int J Radiat Oncol Biol Phys. 2018. PMID: 31200811 No abstract available.
References
-
- Gillison ML, D’Souza G, Westra WH, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst 2008; 100: 407–420. - PubMed
-
- Weinberger PM, Yu Z, Haffty BG, et al. Molecular classification identifies a subset of human papillomavirus--associated oropharyngeal cancers with favorable prognosis. J Clin Oncol 2006; 24: 736–747. - PubMed
-
- Klussmann JP, Mooren JJ, Lehnen M, et al. Genetic signatures of HPV-related and unrelated oropharygneal carcinoma and their prognostic implications. Clin Cancer Res 2009; 15: 1179–1786. - PubMed
-
- Lassen P, Eriksen JG, Hamilton S, et al. Effect of HPV-associated p16 expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol 2009; 27: 1992–1998. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

