Dose-dependent T-cell Dynamics and Cytokine Cascade Following rVSV-ZEBOV Immunization
- PMID: 28434944
- PMCID: PMC5440606
- DOI: 10.1016/j.ebiom.2017.03.045
Dose-dependent T-cell Dynamics and Cytokine Cascade Following rVSV-ZEBOV Immunization
Abstract
Background: The recent West African Ebola epidemic led to accelerated efforts to test Ebola vaccine candidates. As part of the World Health Organisation-led VSV Ebola Consortium (VEBCON), we performed a phase I clinical trial investigating rVSV-ZEBOV (a recombinant vesicular stomatitis virus-vectored Ebola vaccine), which has recently demonstrated protection from Ebola virus disease (EVD) in phase III clinical trials and is currently in advanced stages of licensing. So far, correlates of immune protection are incompletely understood and the role of cell-mediated immune responses has not been comprehensively investigated to date.
Methods: We recruited 30 healthy subjects aged 18-55 into an open-label, dose-escalation phase I trial testing three doses of rVSV-ZEBOV (3×105 plaque-forming units (PFU), 3×106 PFU, 2×107 PFU) (ClinicalTrials.gov; NCT02283099). Main study objectives were safety and immunogenicity, while exploratory objectives included lymphocyte dynamics, cell-mediated immunity and cytokine networks, which were assessed using flow cytometry, ELISpot and LUMINEX assay.
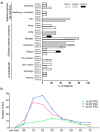
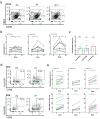
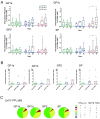
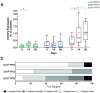
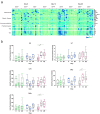
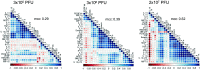
Findings: Immunization with rVSV-ZEBOV was well tolerated without serious vaccine-related adverse events. Ebola virus-specific neutralizing antibodies were induced in nearly all individuals. Additionally, vaccinees, particularly within the highest dose cohort, generated Ebola glycoprotein (GP)-specific T cells and initiated a cascade of signaling molecules following stimulation of peripheral blood mononuclear cells with Ebola GP peptides.
Interpretation: In addition to a benign safety and robust humoral immunogenicity profile, subjects immunized with 2×107 PFU elicited higher cellular immune responses and stronger interlocked cytokine networks compared to lower dose groups. To our knowledge these data represent the first detailed cell-mediated immuneprofile of a clinical trial testing rVSV-ZEBOV, which is of particular interest in light of its potential upcoming licensure as the first Ebola vaccine. VEBCON trial Hamburg, Germany (NCT02283099).
Keywords: Cytokines; Ebola vaccine; Humoral and cell-mediated immune responses; Phase I study; T-cell responses; rVSV-ZEBOV.
Copyright © 2017. Published by Elsevier B.V.
Figures


References
-
- Agnandji S.T., Huttner A., Zinser M.E., Njuguna P., Dahlke C., Fernandes J.F., Yerly S., Dayer J.A., Kraehling V., Kasonta R., Adegnika A.A., Altfeld M., Auderset F., Bache E.B., Biedenkopf N., Borregaard S., Brosnahan J.S., Burrow R., Combescure C., Desmeules J., Eickmann M., Fehling S.K., Finckh A., Goncalves A.R., Grobusch M.P., Hooper J., Jambrecina A., Kabwende A.L., Kaya G., Kimani D., Lell B., Lemaitre B., Lohse A.W., Massinga-Loembe M., Matthey A., Mordmuller B., Nolting A., Ogwang C., Ramharter M., Schmidt-Chanasit J., Schmiedel S., Silvera P., Stahl F.R., Staines H.M., Strecker T., Stubbe H.C., Tsofa B., Zaki S., Fast P., Moorthy V., Kaiser L., Krishna S., Becker S., Kieny M.P., Bejon P., Kremsner P.G., Addo M.M., Siegrist C.A. Phase 1 trials of rVSV Ebola vaccine in Africa and Europe. N. Engl. J. Med. 2016;374:1647–1660. - PMC - PubMed
-
- Blom K., Braun M., Ivarsson M.A., Gonzalez V.D., Falconer K., Moll M., Ljunggren H.G., Michaelsson J., Sandberg J.K. Temporal dynamics of the primary human T cell response to yellow fever virus 17D as it matures from an effector- to a memory-type response. J. Immunol. 2013;190:2150–2158. - PubMed
-
- Dahlke C., Lunemann S., Kasonta R., Kreuels B., Schmiedel S., Ly M.L., Fehling S.K., Strecker T., Becker S., Altfeld M., Sow A., Lohse A.W., Munoz-Fontela C., Addo M.M. Comprehensive characterization of cellular immune responses following Ebola virus infection. J Infect Dis. 2017;215(2):287–292. (PMID: 27799354) - PubMed
-
- De Santis O., Audran R., Pothin E., Warpelin-Decrausaz L., Vallotton L., Wuerzner G., Cochet C., Estoppey D., Steiner-Monard V., Lonchampt S., Thierry A.C., Mayor C., Bailer R.T., Mbaya O.T., Zhou Y., Ploquin A., Sullivan N.J., Graham B.S., Roman F., De Ryck I., Ballou W.R., Kieny M.P., Moorthy V., Spertini F., Genton B. Safety and immunogenicity of a chimpanzee adenovirus-vectored Ebola vaccine in healthy adults: a randomised, double-blind, placebo-controlled, dose-finding, phase 1/2a study. Lancet Infect. Dis. 2016;16:311–320. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

