Cross-talk between sumoylation and phosphorylation in mouse spermatocytes
- PMID: 28435066
- PMCID: PMC5497584
- DOI: 10.1016/j.bbrc.2017.04.107
Cross-talk between sumoylation and phosphorylation in mouse spermatocytes
Abstract
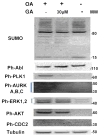
The meiotic G2/M1 transition is mostly regulated by posttranslational modifications, however, the cross-talk between different posttranslational modifications is not well-understood, especially in spermatocytes. Sumoylation has emerged as a critical regulatory event in several developmental processes, including reproduction. In mouse oocytes, inhibition of sumoylation caused various meiotic defects and led to aneuploidy. However, the role of sumoylation in male reproduction has only begun to be elucidated. Given the important role of several SUMO targets (including kinases) in meiosis, in this study, the role of sumoylation was addressed by monitoring the G2/M1 transition in pachytene spermatocytes in vitro upon inhibition of sumoylation. Furthermore, to better understand the cross-talk between sumoylation and phosphorylation, the activity of several kinases implicated in meiotic progression was also assessed upon down-regulation of sumoylation. The results of the analysis demonstrate that inhibition of sumoylation with ginkgolic acid (GA) arrests the G2/M1 transition in mouse spermatocytes preventing chromosome condensation and disassembling of the synaptonemal complex. Our results revealed that the activity of PLK1 and the Aurora kinases increased during the G2/M1 meiotic transition, but was negatively regulated by the inhibition of sumoylation. In the same experiment, the activity of c-Abl, the ERKs, and AKT were not affected or increased after GA treatment. Both the AURKs and PLK1 appear to be "at the right place, at the right time" to at least, in part, explain the meiotic arrest obtained in the spermatocyte culture.
Keywords: AURKs; Ginkgolic acid; Meiosis; PLK1; Phosphorylation; Sumoylation.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures


References
-
- Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2001;2(4):280–91. - PubMed
-
- Liu D, et al. Cyclin A1 is required for meiosis in the male mouse. Nat Genet. 1998;20(4):377–80. - PubMed
-
- Kimmins S, et al. Differential functions of the Aurora-B and Aurora-C kinases in mammalian spermatogenesis. Mol Endocrinol. 2007;21(3):726–39. - PubMed
-
- Tarsounas M, Pearlman RE, Moens PB. Meiotic activation of rat pachytene spermatocytes with okadaic acid: the behaviour of synaptonemal complex components SYN1/SCP1 and COR1/SCP3. J Cell Sci. 1999;112(Pt 4):423–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

