Magnetization Transfer Contrast and Chemical Exchange Saturation Transfer MRI. Features and analysis of the field-dependent saturation spectrum
- PMID: 28435103
- PMCID: PMC5650949
- DOI: 10.1016/j.neuroimage.2017.04.045
Magnetization Transfer Contrast and Chemical Exchange Saturation Transfer MRI. Features and analysis of the field-dependent saturation spectrum
Abstract
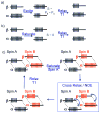
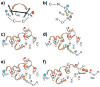
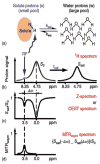
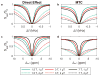
Magnetization Transfer Contrast (MTC) and Chemical Exchange Saturation Transfer (CEST) experiments measure the transfer of magnetization from molecular protons to the solvent water protons, an effect that becomes apparent as an MRI signal loss ("saturation"). This allows molecular information to be accessed with the enhanced sensitivity of MRI. In analogy to Magnetic Resonance Spectroscopy (MRS), these saturation data are presented as a function of the chemical shift of participating proton groups, e.g. OH, NH, NH2, which is called a Z-spectrum. In tissue, these Z-spectra contain the convolution of multiple saturation transfer effects, including nuclear Overhauser enhancements (NOEs) and chemical exchange contributions from protons in semi-solid and mobile macromolecules or tissue metabolites. As a consequence, their appearance depends on the magnetic field strength (B0) and pulse sequence parameters such as B1 strength, pulse shape and length, and interpulse delay, which presents a major problem for quantification and reproducibility of MTC and CEST effects. The use of higher B0 can bring several advantages. In addition to higher detection sensitivity (signal-to-noise ratio, SNR), both MTC and CEST studies benefit from longer water T1 allowing the saturation transferred to water to be retained longer. While MTC studies are non-specific at any field strength, CEST specificity is expected to increase at higher field because of a larger chemical shift dispersion of the resonances of interest (similar to MRS). In addition, shifting to a slower exchange regime at higher B0 facilitates improved detection of the guanidinium protons of creatine and the inherently broad resonances of the amine protons in glutamate and the hydroxyl protons in myoinositol, glycogen, and glucosaminoglycans. Finally, due to the higher mobility of the contributing protons in CEST versus MTC, many new pulse sequences can be designed to more specifically edit for CEST signals and to remove MTC contributions.
Keywords: CEST; MTC; Magnetization transfer; NOE; Nuclear Overhauser enhancement.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
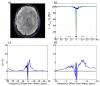
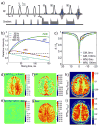
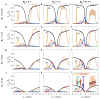
Similar articles
-
Magnetization transfer contrast-suppressed imaging of amide proton transfer and relayed nuclear overhauser enhancement chemical exchange saturation transfer effects in the human brain at 7T.Magn Reson Med. 2016 Jan;75(1):88-96. doi: 10.1002/mrm.25990. Epub 2015 Oct 7. Magn Reson Med. 2016. PMID: 26445350 Free PMC article.
-
Variable delay multi-pulse train for fast chemical exchange saturation transfer and relayed-nuclear overhauser enhancement MRI.Magn Reson Med. 2014 May;71(5):1798-812. doi: 10.1002/mrm.24850. Epub 2013 Jun 27. Magn Reson Med. 2014. PMID: 23813483 Free PMC article.
-
Nuclear Overhauser enhancement (NOE) imaging in the human brain at 7T.Neuroimage. 2013 Aug 15;77:114-24. doi: 10.1016/j.neuroimage.2013.03.047. Epub 2013 Apr 6. Neuroimage. 2013. PMID: 23567889 Free PMC article.
-
The relayed nuclear Overhauser effect in magnetization transfer and chemical exchange saturation transfer MRI.NMR Biomed. 2023 Jun;36(6):e4778. doi: 10.1002/nbm.4778. Epub 2022 Jun 20. NMR Biomed. 2023. PMID: 35642102 Free PMC article. Review.
-
CEST, ASL, and magnetization transfer contrast: How similar pulse sequences detect different phenomena.Magn Reson Med. 2018 Oct;80(4):1320-1340. doi: 10.1002/mrm.27341. Epub 2018 May 30. Magn Reson Med. 2018. PMID: 29845640 Free PMC article. Review.
Cited by
-
The cellular heat shock response monitored by chemical exchange saturation transfer MRI.Sci Rep. 2020 Jul 6;10(1):11118. doi: 10.1038/s41598-020-68022-1. Sci Rep. 2020. PMID: 32632120 Free PMC article.
-
Detection of electrostatic molecular binding using the water proton signal.Magn Reson Med. 2022 Aug;88(2):901-915. doi: 10.1002/mrm.29230. Epub 2022 Apr 5. Magn Reson Med. 2022. PMID: 35394084 Free PMC article.
-
3D gradient echo snapshot CEST MRI with low power saturation for human studies at 3T.Magn Reson Med. 2019 Apr;81(4):2412-2423. doi: 10.1002/mrm.27569. Epub 2018 Nov 15. Magn Reson Med. 2019. PMID: 30431179 Free PMC article.
-
Separating fast and slow exchange transfer and magnetization transfer using off-resonance variable-delay multiple-pulse (VDMP) MRI.Magn Reson Med. 2018 Oct;80(4):1568-1576. doi: 10.1002/mrm.27111. Epub 2018 Feb 5. Magn Reson Med. 2018. PMID: 29405374 Free PMC article.
-
A vision of 14 T MR for fundamental and clinical science.MAGMA. 2023 Apr;36(2):211-225. doi: 10.1007/s10334-023-01081-3. Epub 2023 Apr 10. MAGMA. 2023. PMID: 37036574 Free PMC article. Review.
References
-
- Aime S, Castelli DD, Crich SG, Gianolio E, Terreno E. Pushing the sensitivity envelope of lanthanide-based magnetic resonance imaging (MRI) contrast agents for molecular imaging applications. Acc Chem Res. 2009;42:822–831. - PubMed
-
- Balaban RS, Ceckler TL. Magnetization transfer contrast in magnetic resonance imaging. Magn Reson Q. 1992;8:116–137. - PubMed
-
- Behar KL, Rothman DL, Spencer DD, Petroff OA. Analysis of macromolecule resonances in 1H NMR spectra of human brain. Magn Reson Med. 1994;32:294–302. - PubMed
-
- Bottomley PA. MRS Studies of Creatine Kinase Metabolism in Human Heart. eMagRes. 2016;5:1183–1202. doi:1110.1002/9780470034590.emrstm9780470031488.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

