SL2B aptamer and folic acid dual-targeting DNA nanostructures for synergic biological effect with chemotherapy to combat colorectal cancer
- PMID: 28435250
- PMCID: PMC5388264
- DOI: 10.2147/IJN.S132929
SL2B aptamer and folic acid dual-targeting DNA nanostructures for synergic biological effect with chemotherapy to combat colorectal cancer
Abstract
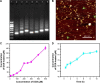
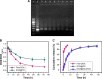
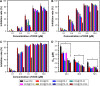
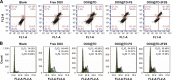
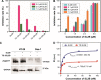
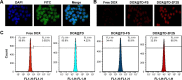
DNA nanostructures prepared by self-assembly possess good stability, high biocompatibility, and low immunogenicity as drug delivery vehicles. In this work, DNA tetrahedron (TD) was constructed and modified with SL2B aptamer (S) and folic acid (F). TD possessed a small diameter (~6 nm) and entered into the nucleus quickly. SL2B aptamer can inhibit cancer cell growth by disturbing vascular endothelial growth factor/Notch signaling pathways. To explore the effect of SL2B number on colorectal cancer inhibition, SL2B multimers (dimer, trimer, and tetramer) were constructed by functionalization of TD with different numbers of SL2B. One SL2B per TD was the most efficient anticancer strategy and showed significantly better anticancer efficacy than SL2B, probably due to the enhanced stability of SL2B by TD. Doxorubicin (DOX) is a potent anticancer agent that can intercalate into DNA double strands. Results showed that TD could facilitate DOX entrance into the nucleus and the intracellular delivery of DOX was further enhanced by functionalization of SL2B and F. DOX-intercalated TD modified with two F and two S (DOX@TD-2F2S) could cause sufficient HT-29 cell inhibition at a much lower DOX concentration. In sum, DOX@TD-2F2S exhibited a synergic anticancer biological effect with chemotherapy and can be a promising strategy for treating colorectal cancer.
Keywords: DNA tetrahedron; SL2B aptamer; VEGF/Notch; colorectal cancer; doxorubicin; folic acid; synergic biological effect with chemotherapy.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures

Similar articles
-
Multifunctional aptamer-based nanoparticles for targeted drug delivery to circumvent cancer resistance.Biomaterials. 2016 Jun;91:44-56. doi: 10.1016/j.biomaterials.2016.03.013. Epub 2016 Mar 10. Biomaterials. 2016. PMID: 26994877
-
Aptamer-guided DNA tetrahedron as a novel targeted drug delivery system for MUC1-expressing breast cancer cells in vitro.Oncotarget. 2016 Jun 21;7(25):38257-38269. doi: 10.18632/oncotarget.9431. Oncotarget. 2016. PMID: 27203221 Free PMC article.
-
A dual-targeting DNA tetrahedron nanocarrier for breast cancer cell imaging and drug delivery.Talanta. 2018 Mar 1;179:356-363. doi: 10.1016/j.talanta.2017.11.034. Epub 2017 Nov 20. Talanta. 2018. PMID: 29310244
-
Advancements in Aptamer-Driven DNA Nanostructures for Precision Drug Delivery.Adv Sci (Weinh). 2024 Jul;11(26):e2401617. doi: 10.1002/advs.202401617. Epub 2024 May 7. Adv Sci (Weinh). 2024. PMID: 38713753 Free PMC article. Review.
-
DNA tetrahedron nanostructures for biological applications: biosensors and drug delivery.Analyst. 2017 Sep 8;142(18):3322-3332. doi: 10.1039/c7an01154g. Analyst. 2017. PMID: 28835943 Review.
Cited by
-
Site-specific anchoring aptamer C2NP on DNA origami nanostructures for cancer treatment.RSC Adv. 2018 Jul 23;8(46):26300-26308. doi: 10.1039/c8ra04589e. eCollection 2018 Jul 19. RSC Adv. 2018. PMID: 35541930 Free PMC article.
-
Synergistic Treatment of Tumor by Targeted Biotherapy and Chemotherapy via Site-Specific Anchoring of Aptamers on DNA Nanotubes.Int J Nanomedicine. 2020 Feb 27;15:1309-1320. doi: 10.2147/IJN.S225142. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32161460 Free PMC article.
-
Visualization of the hepatic and renal cell uptake and trafficking of tetrahedral DNA origami in tumour.Cell Prolif. 2024 Aug;57(8):e13643. doi: 10.1111/cpr.13643. Epub 2024 Apr 4. Cell Prolif. 2024. PMID: 38572799 Free PMC article.
-
Dual-targeting inhibition of TNFR1 for alleviating rheumatoid arthritis by a novel composite nucleic acid nanodrug.Int J Pharm X. 2023 Jan 10;5:100162. doi: 10.1016/j.ijpx.2023.100162. eCollection 2023 Dec. Int J Pharm X. 2023. PMID: 37396624 Free PMC article.
-
Aptamers: novelty tools for cancer biology.Oncotarget. 2018 Jun 1;9(42):26934-26953. doi: 10.18632/oncotarget.25260. eCollection 2018 Jun 1. Oncotarget. 2018. PMID: 29928493 Free PMC article. Review.
References
-
- Erben CM, Goodman RP, Turberfield AJ. Single-molecule protein encapsulation in a rigid DNA cage. Angewandte Chemie. 2006;45(44):7414–7417. - PubMed
-
- Goodman RP, Berry RM, Turberfield AJ. The single-step synthesis of a DNA tetrahedron. Chem Commun. 2004;(12):1372–1373. - PubMed
-
- Kim KR, Kim DR, Lee T, et al. Drug delivery by a self-assembled DNA tetrahedron for overcoming drug resistance in breast cancer cells. Chem Commun. 2013;49(20):2010–2012. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

