Comparative study of porous hydroxyapatite/chitosan and whitlockite/chitosan scaffolds for bone regeneration in calvarial defects
- PMID: 28435251
- PMCID: PMC5388207
- DOI: 10.2147/IJN.S131251
Comparative study of porous hydroxyapatite/chitosan and whitlockite/chitosan scaffolds for bone regeneration in calvarial defects
Abstract
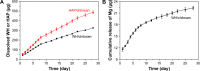
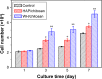
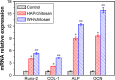
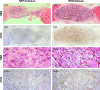
Hydroxyapatite (HAP; Ca10(PO4)6(OH)2) and whitlockite (WH; Ca18Mg2(HPO4)2(PO4)12) are widely utilized in bone repair because they are the main components of hard tissues such as bones and teeth. In this paper, we synthesized HAP and WH hollow microspheres by using creatine phosphate disodium salt as an organic phosphorus source in aqueous solution through microwave-assisted hydrothermal method. Then, we prepared HAP/chitosan and WH/chitosan composite membranes to evaluate their biocompatibility in vitro and prepared porous HAP/chitosan and WH/chitosan scaffolds by freeze drying to compare their effects on bone regeneration in calvarial defects in a rat model. The experimental results indicated that the WH/chitosan composite membrane had a better biocompatibility, enhancing proliferation and osteogenic differentiation ability of human mesenchymal stem cells than HAP/chitosan. Moreover, the porous WH/chitosan scaffold can significantly promote bone regeneration in calvarial defects, and thus it is more promising for applications in tissue engineering such as calvarial repair compared to porous HAP/chitosan scaffold.
Keywords: chitosan; hydroxyapatite; osteogenic differentiation; rat critical calvarial defect; tissue engineering; whitlockite.
Conflict of interest statement
Disclosure The authors declare no conflicts of interest in this work.
Figures








Similar articles
-
In Vitro and In Vivo Evaluation of Whitlockite Biocompatibility: Comparative Study with Hydroxyapatite and β-Tricalcium Phosphate.Adv Healthc Mater. 2016 Jan 7;5(1):128-36. doi: 10.1002/adhm.201400824. Epub 2015 May 12. Adv Healthc Mater. 2016. PMID: 25963732
-
Synergistic interplay between the two major bone minerals, hydroxyapatite and whitlockite nanoparticles, for osteogenic differentiation of mesenchymal stem cells.Acta Biomater. 2018 Mar 15;69:342-351. doi: 10.1016/j.actbio.2018.01.016. Epub 2018 Feb 13. Acta Biomater. 2018. PMID: 29366976 Free PMC article.
-
Porous Chitosan/Nano-Hydroxyapatite Composite Scaffolds Incorporating Simvastatin-Loaded PLGA Microspheres for Bone Repair.Cells Tissues Organs. 2018;205(1):20-31. doi: 10.1159/000485502. Epub 2018 Feb 1. Cells Tissues Organs. 2018. PMID: 29393155
-
Chitosan-based biomaterials for bone tissue engineering.Int J Biol Macromol. 2025 Apr;304(Pt 2):140923. doi: 10.1016/j.ijbiomac.2025.140923. Epub 2025 Feb 11. Int J Biol Macromol. 2025. PMID: 39947561 Review.
-
Chitosan composite with mesenchymal stem cells: Properties, mechanism, and its application in bone regeneration.Int J Biol Macromol. 2024 Aug;275(Pt 1):133502. doi: 10.1016/j.ijbiomac.2024.133502. Epub 2024 Jul 2. Int J Biol Macromol. 2024. PMID: 38960259 Review.
Cited by
-
Chitosan films for regenerative medicine: fabrication methods and mechanical characterization of nanostructured chitosan films.Biophys Rev. 2019 Oct;11(5):807-815. doi: 10.1007/s12551-019-00591-6. Epub 2019 Sep 16. Biophys Rev. 2019. PMID: 31529358 Free PMC article. Review.
-
Chitosan-Based Biomaterial Scaffolds for the Repair of Infected Bone Defects.Front Bioeng Biotechnol. 2022 May 4;10:899760. doi: 10.3389/fbioe.2022.899760. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35600891 Free PMC article. Review.
-
Valproic acid prevents glucocorticoid‑induced osteonecrosis of the femoral head of rats.Int J Mol Med. 2018 Jun;41(6):3433-3447. doi: 10.3892/ijmm.2018.3534. Epub 2018 Mar 6. Int J Mol Med. 2018. PMID: 29512684 Free PMC article.
-
Hydrogel Scaffolds: Towards Restitution of Ischemic Stroke-Injured Brain.Transl Stroke Res. 2019 Feb;10(1):1-18. doi: 10.1007/s12975-018-0655-6. Epub 2018 Aug 27. Transl Stroke Res. 2019. PMID: 30151667 Review.
-
Chitosan alchemy: transforming tissue engineering and wound healing.RSC Adv. 2024 Jun 17;14(27):19219-19256. doi: 10.1039/d4ra01594k. eCollection 2024 Jun 12. RSC Adv. 2024. PMID: 38887635 Free PMC article. Review.
References
-
- Gazdag AR, Lane JM, Glaser D, Forster RA. Alternatives to autogenous bone graft: efficacy and indications. J Am Acad Orthop Surg. 1995;3(1):1–8. - PubMed
-
- Krause F, Younger A, Weber M. Recombinant human BMP-2 and allograft compared with autogenous bone graft for reconstruction of diaphyseal tibial fractures with cortical defects. J Bone Joint Surg Am. 2008;90(5):1168–1169. - PubMed
-
- Thorfve A, Lindahl C, Xia W, et al. Hydroxyapatite coating affects the Wnt signaling pathway during peri-implant healing in vivo. Acta Biomater. 2014;10(3):1451–1462. - PubMed
-
- Wang H, Zhao S, Xiao W, et al. Three-dimensional zinc incorporated borosilicate bioactive glass scaffolds for rodent critical-sized calvarial defects repair and regeneration. Colloids Surf B Biointerfaces. 2015;130:149–156. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

