Recipient Glycemic Micro-environments Govern Therapeutic Effects of Mesenchymal Stem Cell Infusion on Osteopenia
- PMID: 28435461
- PMCID: PMC5399589
- DOI: 10.7150/thno.18181
Recipient Glycemic Micro-environments Govern Therapeutic Effects of Mesenchymal Stem Cell Infusion on Osteopenia
Abstract
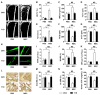
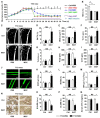
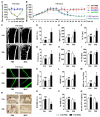
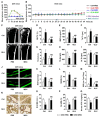
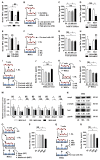
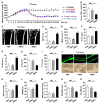
Therapeutic effects of mesenchymal stem cell (MSC) infusion have been revealed in various human disorders, but impacts of diseased micro-environments are only beginning to be noticed. Donor diabetic hyperglycemia is reported to impair therapeutic efficacy of stem cells. However, whether recipient diabetic condition also affects MSC-mediated therapy is unknown. We and others have previously shown that MSC infusion could cure osteopenia, particularly in ovariectomized (OVX) mice. Here, we discovered impaired MSC therapeutic effects on osteopenia in recipient type 1 diabetes (T1D). Through intensive glycemic control by daily insulin treatments, therapeutic effects of MSCs on osteopenia were maintained. Interestingly, by only transiently restoration of recipient euglycemia using single insulin injection, MSC infusion could also rescue T1D-induced osteopenia. Conversely, under recipient hyperglycemia induced by glucose injection in OVX mice, MSC-mediated therapeutic effects on osteopenia were diminished. Mechanistically, recipient hyperglycemic micro-environments reduce anti-inflammatory capacity of MSCs in osteoporotic therapy through suppressing MSC interaction with T cells via the Adenosine monophosphate-activated protein kinase (AMPK) pathway. We further revealed in diabetic micro-environments, double infusion of MSCs ameliorated osteopenia by anti-inflammation, attributed to the first transplanted MSCs which normalized the recipient glucose homeostasis. Collectively, our findings uncover a previously unrecognized role of recipient glycemic conditions controlling MSC-mediated therapy, and unravel that fulfillment of potent therapeutic effects of MSCs requires tight control of recipient micro-environments.
Keywords: Mesenchymal stem cells; anti-inflammation.; cell therapy; glycemic micro-environment; osteopenia; recipient.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
References
-
- Kharaziha P, Hellstrom PM, Noorinayer B, Farzaneh F, Aghajani K, Jafari F. et al. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I-II clinical trial. Eur J Gastroenterol Hepatol. 2009;21:1199–205. - PubMed
-
- Duijvestein M, Vos AC, Roelofs H, Wildenberg ME, Wendrich BB, Verspaget HW. et al. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn's disease: results of a phase I study. Gut. 2010;59:1662–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

