Opioid Receptor Activity and Analgesic Potency of DPDPE Peptide Analogues Containing a Xylene Bridge
- PMID: 28435535
- PMCID: PMC5392763
- DOI: 10.1021/acsmedchemlett.7b00044
Opioid Receptor Activity and Analgesic Potency of DPDPE Peptide Analogues Containing a Xylene Bridge
Abstract
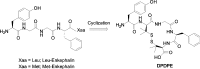
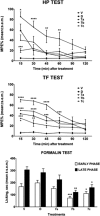
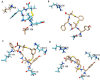
d-Pen2,d-Pen5 enkephalin (DPDPE) is one of the most selective synthetic peptide agonists targeting the δ-opioid receptor. Three cyclic analogues of DPDPE containing a xylene bridge in place of disulfide bond have been synthesized and fully characterized as opioid receptors agonists. The in vitro activity was investigated showing a good affinity of 7a-c for μ- and δ-receptors. In vivo biological assays revealed that 7b is the most potent analogue with the ability to maintain high level of analgesia from 15 to 60 min following intracerebroventricular (i.c.v.) administration, whereas DPDPE was slightly active until 45 min. Compound 7b induced long lasting analgesia also after subcutaneous administration, whereas DPDPE was inactive.
Keywords: DPDPE; Opioids; antinociception; peptides; xylene bridge.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Topographically designed analogues of [D-Pen,D-Pen5]enkephalin.J Med Chem. 1991 Jun;34(6):1823-30. doi: 10.1021/jm00110a010. J Med Chem. 1991. PMID: 1648137
-
Delta opioid receptor enhancement of mu opioid receptor-induced antinociception in spinal cord.J Pharmacol Exp Ther. 1998 Jun;285(3):1181-6. J Pharmacol Exp Ther. 1998. PMID: 9618421
-
Assessment of stereoselectivity of trimethylphenylalanine analogues of delta-opioid [D-Pen(2),D-Pen(5)]-enkephalin.J Neurochem. 2000 Jul;75(1):424-35. doi: 10.1046/j.1471-4159.2000.0750424.x. J Neurochem. 2000. PMID: 10854288
-
Role of mu and delta receptors in the supraspinal and spinal analgesic effects of [D-Pen2, D-Pen5]enkephalin in the mouse.J Pharmacol Exp Ther. 1987 May;241(2):393-400. J Pharmacol Exp Ther. 1987. PMID: 3033214
-
Different types of opioid receptors mediating analgesia induced by morphine, DAMGO, DPDPE, DADLE and beta-endorphin in mice.Naunyn Schmiedebergs Arch Pharmacol. 1990 Jul;342(1):67-71. doi: 10.1007/BF00178974. Naunyn Schmiedebergs Arch Pharmacol. 1990. PMID: 1976234
Cited by
-
Characterization of a Nonselective Opioid Receptor Functional Antagonist: Implications for Development as a Novel Opioid Dependence Medication.ACS Pharmacol Transl Sci. 2024 Feb 12;7(3):654-666. doi: 10.1021/acsptsci.3c00262. eCollection 2024 Mar 8. ACS Pharmacol Transl Sci. 2024. PMID: 38481688 Free PMC article.
-
Cyclizing Painkillers: Development of Backbone-Cyclic TAPS Analogs.Front Chem. 2020 Nov 12;8:532577. doi: 10.3389/fchem.2020.532577. eCollection 2020. Front Chem. 2020. PMID: 33282822 Free PMC article.
-
Cyclic Biphalin Analogues Incorporating a Xylene Bridge: Synthesis, Characterization, and Biological Profile.ACS Med Chem Lett. 2017 Jul 12;8(8):858-863. doi: 10.1021/acsmedchemlett.7b00210. eCollection 2017 Aug 10. ACS Med Chem Lett. 2017. PMID: 28835802 Free PMC article.
-
Discovery of Orexant and Anorexant Agents with Indazole Scaffold Endowed with Peripheral Antiedema Activity.Biomolecules. 2019 Sep 16;9(9):492. doi: 10.3390/biom9090492. Biomolecules. 2019. PMID: 31527522 Free PMC article.
-
On resin click-chemistry-mediated synthesis of novel enkephalin analogues with potent anti-nociceptive activity.Sci Rep. 2019 Apr 8;9(1):5771. doi: 10.1038/s41598-019-42289-5. Sci Rep. 2019. PMID: 30962495 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Research Materials

