The complex legal and ethical issues related to generic medications. Viral hepatitis: a case study
- PMID: 28435690
- PMCID: PMC5384269
- DOI: 10.1016/S2055-6640(20)30286-7
The complex legal and ethical issues related to generic medications. Viral hepatitis: a case study
Abstract
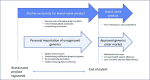
The economic impact of medications is significant, with many countries unable to afford the essential medicines listed by the WHO. Generic medications are one strategy to address this issue. Generic medications are similar to but not the same as originator medications. They have a significant cost advantage because they do not require the background research and development studies to support registration. Consequently, they are gaining increased market share in both the developed and developing world. Many new medications are now licensed to generic manufacturers in the developing world. As a result, it is possible for patients to bypass regulatory and cost barriers by importing medications directly from generic producers. Importation of the novel hepatitis C direct-acting antiviral therapy into Australia before it was registered in the country is an illustrative case study. This review will characterise generic medications and some of the legal and ethical issues around their utilisation, focusing on the relevant players, including pharma, government, patients and doctors.
Figures
Similar articles
-
Generic medications for hepatitis C.Liver Int. 2016 Jul;36(7):925-8. doi: 10.1111/liv.13120. Liver Int. 2016. PMID: 27306302 Review.
-
The use of generic medications for hepatitis C.Liver Int. 2016 Jul;36(7):929-32. doi: 10.1111/liv.13157. Liver Int. 2016. PMID: 27306303 Free PMC article.
-
The market dynamics of generic medicines in the private sector of 19 low and middle income countries between 2001 and 2011: a descriptive time series analysis.PLoS One. 2013 Sep 30;8(9):e74399. doi: 10.1371/journal.pone.0074399. eCollection 2013. PLoS One. 2013. PMID: 24098644 Free PMC article.
-
Bioequivalent pharmacokinetics for generic and originator hepatitis C direct-acting antivirals.J Virus Erad. 2018 Apr 1;4(2):128-131. doi: 10.1016/S2055-6640(20)30257-0. J Virus Erad. 2018. PMID: 29682307 Free PMC article.
-
Generic medicines: issues and relevance for global health.Fundam Clin Pharmacol. 2015 Dec;29(6):529-42. doi: 10.1111/fcp.12155. Epub 2015 Oct 12. Fundam Clin Pharmacol. 2015. PMID: 26405851 Review.
Cited by
-
Pharmacokinetic equivalence study of nonsteroidal anti-inflammatory drug etoricoxib.Clin Pharmacol. 2018 Apr 6;10:43-51. doi: 10.2147/CPAA.S161024. eCollection 2018. Clin Pharmacol. 2018. PMID: 29670410 Free PMC article.
References
-
- WHO Essential medicines and health products. 2017. Available at: www.who.int/medicines/services/essmedicines_def/en/ ( accessed February 2017).
-
- WHO The World Medicines Situation Report. WHO; 2011. Available at: www.who.int/medicines/areas/policy/world_medicines_situation/en/ ( accessed February 2017).
-
- FDA Facts about generic drugs. 2016. Available at: www.fda.gov/downloads/drugs/resourcesforyou/consumers/buyingusingmedicin... ( accessed February 2017).
-
- GPhA Generic Pharmaceutical Association Generic Drug Savings in the US. Washington DC, USA: GPhA; 2013. Available at: www.gphaonline.org/media/cms/2013_Savings_Study_12.19.2013_FINAL.pdf ( accessed March 2017).
-
- Tourncure Stop the wait. 2017. Available at: www.tourncure.com ( accessed March 2017).
Publication types
LinkOut - more resources
Full Text Sources
Research Materials